CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
This clinical article aims to discuss different irrigation components in conjunction with the use of NaOCl.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Identify some of the favorable qualities and properties of sodium hypochlorite (NaOCl).
- Identify various components of endodontic irrigation.
- Recognize some basic biologic objectives of root canal treatment.
- Realize different irrigation components in conjunction with the use of NaOCl.
- Recognize some ideal properties of an irrigant.
Dr. Jeffrey Krupp discusses different irrigation components in conjunction with the use of NaOCl
Sodium hypochlorite (NaOCl) is the most ubiquitous irrigating solution in endodontics. It has numerous favorable qualities and properties. NaOCl performs bactericidal cytotoxicity, benefits the dissolution of organic material, and enhances minor lubrication.1 Sodium hypochlorite by itself is not sufficient for total cleaning of the endodontic system.2 It has no measurable effect on the smear layer, and its high surface tension does not allow for cleaning and disinfection of the root canal system’s totality. For this reason, and according to individual clinical situations, we must use other irrigants in combination with sodium hypochlorite.
The basic biologic objective of root canal treatment, beyond removal of subjective symptomatology, relies on the complete removal of the pulpal tissue and the destruction of residual microorganisms, including yeasts, molds, and viruses often found in infected root canals. Therapeutic endodontic treatment also mandates an effective radicular seal in order to prevent recolonization of the root canal system with microorganisms.3
Dr. Herbert Schilder emphasized the importance of cleaning and shaping. We as clinicians respect that the proper shaping of a canal develops a conduit, which can now be effectively irrigated and cleansed.4
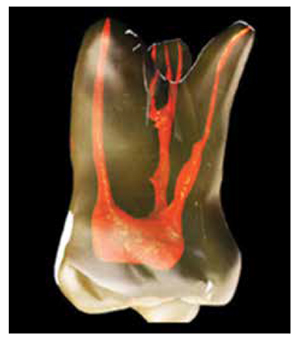
The components of endodontic irrigation fall into a number of categories. Irrigation solutions, devices, protocols, advances, and clinical challenges all factor to some degree how the clinician approaches this foundational aspect of endodontic therapy. The choice of irrigants will always vary from practitioner to practitioner. No irrigant to date provides 100% elimination of bacteria and cleansing of the root canal system.
This article will review in part different irrigation components in conjunction with the use of NaOCl, a prominent endodontic irrigant.
The amazing complexity of human dental morphology has been described and extensively studied. The astounding variation of root and canal morphology renders therapeutic endodontic treatments a constant challenge.5,6
The intricacy and anatomical complexity of the root canal system, presence of dentinal tubules comprising the root morphology, invasion of the tubules by microorganisms, formation of smear layer during instrumentation, and presence of dentin as a tissue are the major challenges in obtaining the therapeutic intention of complete cleaning and shaping of root canal systems.7

Besides sodium hypochlorite (NaOCl), other commonly used irrigants are chlorhexidine, ethylenediamine tetraaceticacid (EDTA), and a mixture of tetracycline, an acid, and a detergent (MTAD).
Sodium hypochlorite is an excellent non-specific proteolytic and antimicrobial irrigation solution. It is effective against most bacteria typically found in the canal system, including Enterococcus faecalis but is concentration dependent.8
Although sodium hypochlorite appears to be the most prominent single endodontic irrigant, it displays no effect on the smear layer, and its high surface tension does not allow for cleaning and disinfection of the root canal system’s totality. Therefore, according to individual clinical situations, we must use other irrigants in combination with sodium hypochlorite.9,10
Chelating agents such as ethylenediamine tetraacetic acid (EDTA)11 and citric acid12 have therefore been recommended as adjuvants in root canal therapy. In addition to their cleaning ability, chelators may detach biofilms adhering to root canal walls.
Research has reported an increase in the antibacterial effect of 5.0% NaOCl when used alternately with 10% EDTA solution. This is related to the demineralizing action of EDTA, which prevents smear layer formation during instrumentation, resulting in an increased NaOCl penetration into the dentinal tubules.13
Citric acid has been reported to affect the removal of the smear layer similar to that obtained by EDTA. Citric acid is less cyto-toxically irritable to tissue than EDTA.14
Ideal properties of an irrigant15
- Tissue/debris solvent
- Low toxicity
- Low surface tension
- Lubricant
- Sterilization/disinfection
- Removal of smear layer
- Have a broad antimicrobial spectrum and high efficacy against anaerobic and facultative microorganisms organized within biofilms
- Inactivate endotoxin
- Systemically nontoxic, non-caustic to periodontal tissues
- Low cost, easy availability, shelf life
Sodium hypochlorite when used as an endodontic irrigant becomes an effective antimicrobial with tissue-dissolving capabilities. It has low viscosity, allowing easy introduction into the canal, an acceptable shelf life, easy availability, and inexpensive. The antibacterial and tissue dissolution action of hypochlorite increases with its concentration, but this accompanies an increase in toxicity.
Concentrations ranging from 0.5% – 5.25% are widely used. Although less concentrated solutions have shown antimicrobial effectiveness, higher concentrations of NaOCI present faster and greater bactericidal effect. However, the higher the concentration of NaOCl, the greater its cytotoxic effect.16,17
Different contact times evaluated both at 1 minute and 5 minutes were found to be effective against growth of E. faecalis. Retamozo, et al.,18 reported that long exposure time to NaOCl is needed for elimination of E. faecalis-contaminated dentin, such as 40 minutes. As clinicians we need to keep in mind the findings of Zhang, et al.,19 where it was reported that collagen degradation was significantly increased, and the flexural strength of mineralized dentin was significantly reduced after the use of 5.25% NaOCl as the initial irrigant for more than 1 hour.
My office employs “Fresh Scent” Clorox®, which has a concentration of 4.13% directly from the bottle.20
It is always advised to flush the root canals with NaOCl throughout the cleaning and shaping process as it helps to increase the working time available for the irrigant and improves cutting efficiency of the instrument. Additionally, the axiom is never manipulate a file in a dry canal to avoid file separation due to binding of the file in the canal. Keeping irrigant in the canal decreases the potential for file separation as well as helps flush out debris as the file is moved in an apical-coronal direction.
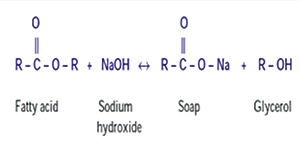
NaOCl neutralizes the amino acids forming water and salt. With the output of hydroxyl ions, a reduction in the pH occurs. The hypochlorous acid, a substance present in the NaOCl solution, when in contact with organic tissue, acts as a solvent releasing chlorine, which combined with the amino group of proteins, forms chloramines. Hypochlorous acid (HOCl) and hypochlorite ions (OCl-) lead to degradation of amino acids and hydrolysis, so the dissolution of organic necrotic tissue can be verified in the saponification reaction when NaOCl degrades fatty acids and lipids, resulting in soap and glycerol and promoting a deodorant effect. Sodium hypochlorite exhibits a dynamic balance as shown by the following reaction:21
Saponification reaction
Sodium hypochlorite acts on fatty acids, transforming them into fatty acid salts (soap) and glycerol (alcohol) that reduce the surface tension of the remaining solution.
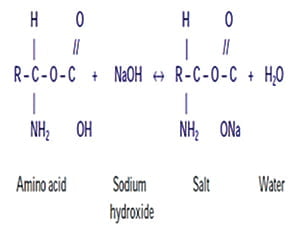
 Neutralization reaction
Neutralization reaction
NaOCl neutralizes amino acids and forms water and salt. With the exit of hydroxyl ions, there is a reduction of ph.
 Chloramination reaction
Chloramination reaction
Hypochlorous acid, present in NaOCl solution, when in contact with organic tissue acts as a solvent, releases chlorine that, combined with the protein amino group, forms chloramines that interfere in cell metabolism, helping to render its antimicrobial affect.
 Hypochlorous acid (HOCl-) and hypochlorite ions (OCl-) lead to amino acid degradation and hydrolysis.
Hypochlorous acid (HOCl-) and hypochlorite ions (OCl-) lead to amino acid degradation and hydrolysis.
Chlorine (strong oxidant) presents antimicrobial action inhibiting bacterial enzymes leading to an irreversible oxidation of SH groups (sulfhydryl group) of essential bacterial enzymes.21
Sodium hypochlorite is a strong base (pH>11). Its antimicrobial mechanism of action can be observed verifying its physio-chemical characteristics and its reaction with organic tissue.21
No irrigant provides 100% elimination of bacteria
and cleansing the root canal. Notwithstanding
the complications, NaOCl is the benchmark
irrigant used in normal clinical practice.
The high pH of sodium hypochlorite interferes in the cytoplasmic membrane integrity with an irreversible enzymatic inhibition, biosynthetic modification of cellular metabolism, and phospholipid degradation observed in lipidic peroxidation. The amino acid chloramination reaction forming chloramines disrupt cellular metabolism. Oxidation enhances irreversible bacterial enzymatic inhibition replacing hydrogen with chlorine. This enzyme inactivation can be visualized with the reaction of chlorine among amino groups (NH2-) and an irreversible oxidation of sulfhydryl groups (SH) of bacterial enzymes (cysteine). Thus, sodium hypochlorite displays antimicrobial activity with action on bacterial essential enzymatic sites encouraging irreversible inactivation originated by hydroxyl ions and chloramination activity. Dissolution of organic tissue can be confirmed in the saponification reaction as sodium hypochlorite damages fatty acids and lipids resulting in soap and glycerol.21
The antimicrobial effectiveness of sodium hypochlorite, based in its high pH (hydroxyl ions action), is similar to the mechanism of action of calcium hydroxide.22
Mechanism of action of sodium hypochlorite is that the free chlorine in NaOCl dissolves vital and necrotic tissue by breaking down proteins into amino acids.23
Sodium hypochlorite has been demonstrated to be an effective agent against a broad spectrum of bacteria and to dissolve vital as well as necrotic tissue.24
Beside their wide-spectrum, nonspecific killing efficacy on all microbes, hypochlorite preparations are sporicidal and virucidal,25 and display enhanced tissue-dissolving effects on necrotic rather than on vital tissues.26
Decreasing the concentration of the NaOCl solution reduces its toxicity, anti-bacterial effect, and ability to dissolve tissues. Increasing the temperature of a less concentrated solution helps in improving its effectiveness. Several studies revealed that warmed NaOCl solutions dissolved organic tissues better and exhibited greater antimicrobial efficacy compared to non-heated solutions.27-31
The ability of NaOCl to dissolve organic tissues is directly proportional to its concentration. According to Baumgartner and Cuenin, efficacy of the solvent and disinfectant action of NaOCl solutions at low concentrations can be increased by using higher volume of solution and frequent exchanges.32
Numerous endodontic protocols have recommended the alternate use of NaOCl and EDTA. The alternate or mixed use of EDTA during instrumentation with 2.5% sodium hypochlorite was the most effective form of irrigation for the removal of smear layer on the cervical and middle thirds. No form of irrigation was sufficiently effective to remove the smear layer in the apical third.33
Berutti, et al.,34 observed an increase in the antibacterial effect of 5.0% NaOCI when used alternately with 10% EDTA solution. This is related to the demineralizing action of EDTA, which prevents smear layer formation during instrumentation, resulting in an increased NaOCI penetration into the dentinal tubules.
Various irrigation techniques are available, but NaOCl is generally delivered to the canal system using an irrigating syringe and tip. A safe-ended/side irrigation tip is recommended as this prevents accidental extrusion of the solution apically. The recommended tip should be passive in the canal and not be used to express it from the syringe as this will also prevent a potential NaOCl accident. Other methods include agitation with brushes and manual dynamic agitation with files or gutta-percha points. The previously mentioned methods are mechanical irrigation techniques. In today’s endodontic environment, rotary irrigation systems are also extensively used such as rotary brushes, continuous irrigation during instrumentation, sonic and ultrasonic vibrations, and application of negative pressure during irrigation of the root canal system.35
It is always advised to flush the root canals with NaOCl throughout the cleaning and shaping process as it helps to increase the working time available for the irrigant and improves cutting efficiency of the instrument.
The major disadvantages of NaOCl are its cytotoxicity when injected into periradicular tissues, unpleasant smell and taste, ability to bleach clothes, and ability to cause corrosion of metal objects.36 In addition, it neither kills all bacteria,37-40 nor removes the entire smear layer.41 It also alters the properties of dentin.42,43
Most of the hypochlorite accidents are due to incorrect determination of endodontic working length, iatrogenic widening of the apical foramen, lateral perforation, or wedging of the irrigating needle. Clearly, precautions must be undertaken to prevent such mishaps.
Chlorhexidine has also been recommended used in conjunction with NaOCl as an irrigant as it raises effectiveness of the irrigation protocol. However, being a highly reactive molecule, it creates problems when used in a multiple-irrigant regimen. When sodium hypochlorite and chlorhexidine are mixed, an orange-brown precipitate known as para-chloroaniline is formed, which might be carcinogenic, although that has not been substantiated. Clinically, it’s seen as a difficult-to-remove, orange-brown film on tooth structure where the reaction occurs. The major advantages of chlorhexidine over NaOCl are its lower cytotoxicity and lack of foul smell and bad taste.44-48
Endodontic success relies on the eradication of microorganisms, integrating removal of the smear layer during cleaning and shaping. Clinical success factors utilizing the NaOCl result in the irrigant’s full potential while treating endodontic cases.
The application of irrigants differs from practitioner to practitioner. No irrigant provides 100% elimination of bacteria and cleansing the root canal. Notwithstanding the complications, NaOCl is the benchmark irrigant used in normal clinical practice. Appropriate administration of the preferred irrigant helps to achieve sufficient antimicrobial effect, thereby enhancing endodontic therapeutic success.
References
- Barnard D, Davies J, Figdor D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J. 1996;29(5):320-326.
- Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endod J. 1999;32(2):99-102.
- Garberoglio R, Becce C. Smear layer removal by root canal irrigants. A comparative scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1994;78(3):359-367
- Schilder, H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18(2) 269-296.
- Hess, W. The anatomy of the root canals of the teeth of the permanent dentition. In: E. Zurcher (Ed.) The anatomy of the root canals of the teeth of the deciduous dentition, and of the first permanent molars. London, John Bale, Sons & Danielson, Ltd; 1925.
- Barrett, M.T. The internal anatomy of the teeth with special reference to the pulp and its branches. Dent Cosmos. 1925; 67:581–592.
- Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2002;94(6):658-666.
- Siqueira JF Jr, Rôças IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26(6):331-334
- Barnard D, Davies J, Figdor D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J. 1996;29(3):320-326.
- Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endo J. 1999;32(2):99-102.
- Nygaard Östby B. Chelation in root canal therapy. Odontol Tidskr. 1957;65:311.
- Loel DA. Use of acid cleanser in endodontic therapy. J Am Dent Assoc. 1975;90(1):148-151.
- Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod. 1997;23(12):725–727.
- Machado-Silveiro Lf, González-Lopez S, González-Rodríguez MP. Decalcification of root canal dentin by citric acid, EDTA and sodium citrate. Int Endod J. 2004;37(6):365-369.
- Schäfer E. Irrigation of the root canal. ENDO. 2007;1(1):11-27.
- Shih M, Marshall FJ, Rosen S. The bactericidal efficiency of sodium hypochlorite as an endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1970;29(4):613-619.
- Marion JJC, Manhães FC, Bajo H, Duque TM. Efficiency of different concentrations of sodium hypochlorite during endodontic treatment. Literature review. Dental Press Endod. 2012;2(4):32-37.
- Retamozo B, Shabahang S, Johnson N, Aprecio RM, Torabinejad M. Minimum contact time and concentration of sodium hypochlorite required to eliminate Enterococcus faecalis. J Endod. 2010;36(3):520-523.
- Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, et al. Effects of different exposure times and concentrations of sodium hypochlorite /ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod. 2010;36(1):105-109.
- Clorox Company, 1221 Broadway, Oakland, California, Oral communication, date.
- Estrela C, Estrela CR, Barbin EL, Spanó JC, Marchesan MA, Pécora JD. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13(2):113-117.
- Estrela C, Sydney GB, Bammann LL, Felippe Júnior O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995; 6(2):85-90.
- Johnson WT, Noblett WC. Cleaning and Shaping In: 4th ed. Saunders. Endodontics: Principles and Practice. Philadelphia, PA, 2009.
- Senia ES, Marraro RV, Mitchell JL. Rapid sterilization of gutta-percha cones with 5.25% sodium hypochlorite. J Endod. 1975;1(4):136-140.
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999; 12(1):147–179.
- Austin JH, Taylor HD. Behavior of hypochlorite and of chloramine-T solutions in contact with necrotic and normal tissue in vivo. J Exp Med. 1918; 27(5):627–633
- Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005; 31(9): 669-671.
- Cunningham WT, Balekjian, AY. Effect of temperature on collagen dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1980; 49(2):175-177.
- Kamburis JJ, Barker TH, Barfield RD, Eleazer PD. Removal of organic debris from bovine dentine shavings. J Endod. 2003; 29(9):559-561.
- Cunningham WT, Joseph SW. Effect of temperature on bactericidal action of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1980; 50(6):569-571.
- Abou-Rass M, Oglesby SW. The effect of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod. 1981; 7(8):376-377.
- Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18(12):605-712.
- Silveira LFN, Silveira CF, Martos J, Suita de Castro LA. Evaluation of the different irrigation regimens with sodium hypochlorite and EDTA in removing the smear layer during root canal preparation. JMAU. 2013; 1(1-2):51–56.
- Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod. 1997; 23(12):725–727.
- Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009; 35(6):791-804.
- Gomes BP, Ferraz CCR, Vianna ME, Berber VB, Teixeira FB, de Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001; 34(6):424-428.
- Siqueira JF Jr., Machado AG, Silveira RM, Lopes HP, de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J. 1997; 30(4):279-82.
- Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997; 30(5):297-306.
- Shuping GB, Ørstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000; 26(12):751-5.
- Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003; 29(9):576-9.
- McCome D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975; 1(7):238-42.
- Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J. 2001; 34(2):120-32.
- Grigoratos D, Knowles J, Ng YL, Gulabivala K. Effect of exposing dentine to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. Int Endod J. 2001;34(2):113-115.
- Carter D. Endodontic Irrigants. Dentaltown.com. https://www.dentaltown.com/Images/dentaltown/magimages/0211/DTFeb11pg80.pdf. Published February 2011. Accessed June 13, 2016.
- Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004; 30(11):785–7.
- Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1:6-Di-4-chlorophenyldiguanidohexane (hibitane); laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol Chemother. 1954; 9(2):192– 196.
- Hennessey TS. Some antibacterial properties of chlorhexidine. J Periodontal Res Suppl. 1973; 12:61–67.
- Emilson CG. Susceptibility of various microorganisms to chlorhexidine. Scand J Dent Res. 1977; 85(4):255–265.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


