CE Expiration Date: September 3, 2024
CEU (Continuing Education Unit):2 Credit(s)
AGD Code: 070
Educational aims and objectives
This self-instructional course for dentists aims to review several available methods for activating irrigants to improve root canal disinfection and endodontic outcomes.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Recognize the limitations of chemomechanical debridement without activation.
- Identify the relationship between canal disinfection and endodontic clinical outcomes.
- Detail the mechanisms of action underlying heat, laser, negative pressure, and acoustic activation of irrigants.
- Compare relative efficacies of activation techniques in categories of tissue dissolution, biofilm disruption, and smear layer removal.
Dr. Eshwar Arasu reviews several methods for root canal disinfection.
Dr. Eshwar Arasu discusses approaches to activating irrigants for effective endodontic treatment
The chemomechanical preparation of the root canal system represents the most important phase of orthograde endodontic treatment. Reliance strictly on mechanical canal opening and fluid chemistry may be insufficient in achieving successful long-term endodontic outcomes. This article was written to review the clinical value of activating endodontic irrigants and the various technologies available to accomplish that task.
Instrumentation of the canal space is intended to create a pathway large enough for the penetration of endodontic irrigants to the apical portion of the root. While hand and rotary files can enable the operator to machine a tunnel that incorporates the main root canal system, mechanical instrumentation alone often fails to address the anatomic complexities of that system. Anastomoses, fins, lateral canals, and apical deltas are common examples of those hard-to-reach morphological traits of root canal systems seen in every tooth type. Failure to adequately debride these mechanically inaccessible areas has been shown to result in endodontic failures.1,2
The chemistry of endodontic irrigants facilitates the removal of microbes and inorganic smear layer that contribute to the disease of endodontics — apical periodontitis. Although a number of chemical agents have been studied and deployed for endodontic treatment, sodium hypochlorite (NaOCl) reigns as the most commonly used irrigant for tissue dissolution and microbial disinfection. While its tissue dissolution efficiency increases commensurate with concentration and contact time, NaOCl solution delivered passively into the canal system via conventional needle irrigation has been shown to be only moderately effective in disrupting biofilms adherent to radicular dentin.3 As seen in the histologic sections of Figure 1 adapted from a study by Ricucci, et al., these resilient biofilms may be the putative etiology for persistent apical disease.

Ethylenediaminetetraacetic acid (EDTA) — a metal-chelating agent — is a common irrigation agent used for removal of the smear layer that serves as a nutritive substrate for intracanal microbes. EDTA also demonstrates limited efficacy in smear layer removal when introduced to the canal system via needle irrigation exclusively. One scanning electron microscopy study assessing root cleanliness of extracted mandibular molars concluded that conventional needle irrigation inadequately addressed the smear layer in the middle and apical thirds of the root.4
The use of intracanal medicaments such as calcium hydroxide in staged root canal treatment can promote pulpal tissue dissolution and canal disinfection. Even with interappointment medication, investigators have identified residual bacteria in apical ramifications, isthmuses, and dentinal tubules.5 If the operator intends to complete endodontic treatment in a single appointment where no unremitting apical drainage is noted, activation of irrigants may be a powerful treatment adjunct capable of facilitating a successful clinical outcome.
Broadly, activation of endodontic irrigants is defined by the application of kinetic energy to procedure fluids. The following content will review activation via 1) thermal heating, 2) laser, 3) negative pressure, and 4) acoustic techniques
Thermal heating
Studies have demonstrated that heating NaOCl solution enables faster pulpal tissue dissolution. Specifically, intracanal hypochlorite warmed to its boiling point via System B™ heat source (Analytic Endodontics) can achieve tissue disintegration approximately 210 times faster than room temperature solution.6
Laser
The use of the laser in endodontics dates back to the 1970s with studies published since then that have reported on the capacity of lasers to vaporize pulpal tissue and disrupt the smear layer.7 Beyond irradiating dentin, investigators have developed laser-activated irrigation (LAI) for tubular dentin disinfection and smear layer removal. LAI technologies available to clinical operators include, but are not limited to, carbon dioxide, mid-infrared erbium (Er:YAG), and neodymium (Nd:YAG) lasers.8,9,10 The bactericidal capability of the laser depends on its wavelength and the resultant thermal effect on disrupting bacterial cell walls. One subset activation technique relies on a Er:YAG laser with subablative energy and short pulses to create intracanal cavitation. In a phenomenon called photon-induced photoacoustic streaming (PIPS), the resultant shockwaves have been shown to disrupt biofilms.3 While most LAI techniques rely on tips advanced in the canal within 5 mm of the apex, PIPS-specific tips are positioned over the orifices and may bypass the need for significant enlarging of the canal spaces with instrumentation. As seen in Figure 2, amended from a study by Lloyd, et al., PIPS permits deep penetration of irrigants into complex pulpal anatomic systems.11
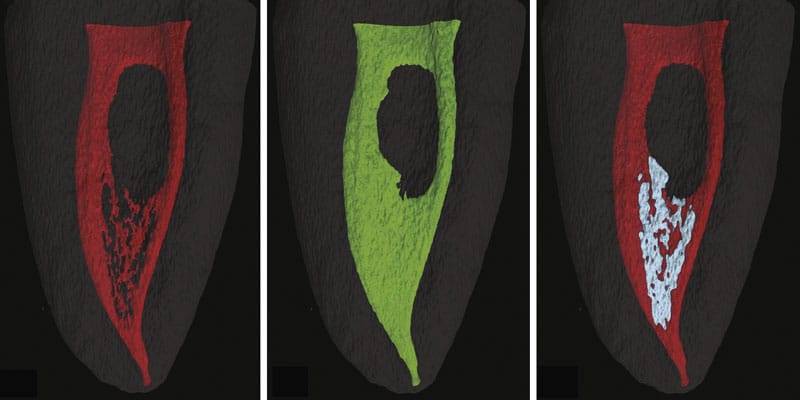
Negative pressure
Positive pressure irrigation relies on delivery of a syringe tip near the working length (WL) and expression of irrigant toward the root apex before evacuation with a suction tip. While this method is common, conventional needle irrigation inadequately disinfects complex canal anatomy and also may lead to hypochlorite extrusion accidents. Negative pressure irrigation, by contrast, begins with irrigant delivery inside the pulp chamber and a narrow cannula suction tip advanced to the WL pulls the fluid into the canal. This approach taken by EndoVac™ (Kerr) has been shown in vitro to possibly mitigate irrigant extrusion.12 However, due to lack of standardization of protocols and laboratory models that may not represent in vivo conditions, comparison for efficacy of negative and positive pressure irrigation has not yielded a definitive victor.13 Potential confounders include irrigation volume, delivery time, and fluid flow rates.
Acoustic techniques
Several clinical technologies acoustically activate endodontic irrigants and do so by operating at select frequencies. Sonic activation (1-8 kHz) has been shown to be capable of removing intraradicular biofilms and debris.14 Moreover, sonic activation of irrigants has been found to yield better disinfection of the canal system relative to manual agitation with endodontic files. Relative to ultrasonic (25-40 kHz) or laser activation, sonic agitation may be less effective in achieving canal disinfection.3 Ultrasonic tips likely facilitate canal debridement via acoustic streaming — the process in which high frequency oscillations lead to mixing of irrigant and shear stresses on radicular dentin walls. One study on comparative safety of intracanal irrigation systems concluded, however, that sonic agitation via EndoActivator™ (Dentsply) yielded less apical extrusion of irrigant relative to ultrasonic activation.15
Multisonic activation — innovated by the GentleWave® System (Sonendo®) — refers to an acoustic field of broadband frequency that occurs as a result of hydrodynamic cavitation in the pulp chamber. This field of sonic energy travels through the fluid into the root canal system, dissolving pulpal tissue, disrupting microbial biofilms, and stripping the smear layer packed into dentinal tubules.16,17 In the system, multisonic activation is also accompanied by fluid degassing and negative pressure, which allow for irrigant penetration into anatomic complexities without obstructive vapor lock and for minimal apical extrusion risk, respectively.18 The synthesis of these fluid dynamic principles allows for safe endodontic treatment of complex, mechanically inaccessible root canal systems with minimal instrumentation as seen in Figures 3A and 3B.

Conclusion
Long-term clinical success in endodontic treatment relies on several important variables, including host immune response, conservation of tooth structure, and coronal seal. The quality of endodontic therapy is largely in the hands of the clinical provider, and adequate disinfection of the root canal system remains a core tenet for treatment success. Discussed in this article are several technologic adjuncts available to clinicians to optimize this critical disinfection phase of treatment. Methods of irrigation activation should be assessed on the merits of safe tissue, biofilm, and smear layer removal efficacy and efficiency throughout the entire root canal system.
For some steps to root canal disinfection with the GentleWave, check out this article by Dr. Rich Mounce:
https://endopracticeus.com/gentlewave-root-canals-cleaned-at-the-speed-of-sound/
References
- Ricucci D, Siqueiria J. Fate of the tissue in lateral canals and apical ramifications in response to pathological conditions and treatment procedures. J Endod. 2010;36(1):1-15.
- Siqueiria J. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34(1):1-10.
- Ordinola-Zapata R, Bramanta C, Aprecio R, Handysides R, Jaramillo D. Biofilm removal by 6% sodium hypochlorite activated by different irrigations techniques. Int Endod J. 2014;47(7):659-666.
- Caron G, Nham K, Bronnec F, Machtou P. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod. 2010;36(8):1361-1366.
- Vera J, Siqueira J, Ricucci D, Loghin S, Fernández N, Flores B, Cruz A. One- versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. J Endod. 2012;38(8):1040-1052.
- Woodmansey K. Intracanal heating of sodium hypochlorite solution: an improved endodontic irrigation technique. Dent Today. 2005;24(10):114-116.
- Weichman J, Johnson F. Laser use in endodontics. A preliminary investigation. Oral Surg Oral Med Oral Pathol. 1971;31(3)416-420.
- Takeda F, Harashima T, Kimura Y, Matstumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999;32(1):32-39.
- Peeters H, Suardita K. Efficacy of smear layer removal at the root tip by using ethylenediaminetetraacetic acid and erbium, chromium: yttrium, scandium, gallium garnet laser. J Endod. 2011;37(11):1585-1589.
- George R, Meyers I, Walsh L. Laser activation of endodontic irrigants with improved conical laser fiber tips for removing smear layer in the apical third of the root canal. J Endod. 2008;34(12):1524-1527.
- Lloyd A, Uhles J, Clement D, Garcia-Godoy F. Elimination of intracanal tissue and debris through a novel laser-activated system assessed using high-resolution micro-computed tomography: a pilot study. J Endod. 2014;40(4):584-587.
- C Boutsioukis, Psimma Z, van der Sluis LW. Factors affecting irrigant extrusion during root canal irrigation: a systemative review. Int Endod J. 2013;46(7):599-618.
- Konstantinidi E, Psimma Z, Chávez de Paz E, Boutsioukis C. Apical negative pressure irrigation versus syringe irrigation: a systematic review of cleaning and disinfection of the root canal system. Int Endod J. 2016;50(11):1034-1054.
- Sabins R, Johnson J, Hellstein J. A comparison of the cleaning efficacy of short-term sonis and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod. 2003;29(10):674-678.
- Desai P, Himel V. Comparative Safety of Various Intracanal Irrigations Systems. J Endod. 2009;35(4):545-549.
- Haapasalo M, Wang Z, Shen Y, et al. Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. J Endod. 2014;40(8):1178-1181.
- Choi H, Park S, Kang M, Shon W. Comparative analysis of biofilm removal efficacy by multisonic ultracleaning system and passive ultrasonic activation. 2019;12:3492.
- Charara K, Friedman S, Sherman A, et al. Assessment of apical extrusion during root canal procedure with the novel GentleWave System in a simulated apical environment. J Endod. 2016;42(1):135-139.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Eshwar Arasu, DMD, MSD is a private practice endodontist in Nashville, Tennessee. Dr. Arasu obtained his dental degree from the Harvard of School Dental Medicine and completed his postdoctoral endodontic training through the residency program at Virginia Commonwealth University. He was awarded a Master of Science in Dentistry for his thesis on the volumetric analysis of surgically treated endodontic lesions via cone beam computed tomography. As of 2019, Dr. Arasu is a Diplomate of the American Board of Endodontics (ABE).
Eshwar Arasu, DMD, MSD is a private practice endodontist in Nashville, Tennessee. Dr. Arasu obtained his dental degree from the Harvard of School Dental Medicine and completed his postdoctoral endodontic training through the residency program at Virginia Commonwealth University. He was awarded a Master of Science in Dentistry for his thesis on the volumetric analysis of surgically treated endodontic lesions via cone beam computed tomography. As of 2019, Dr. Arasu is a Diplomate of the American Board of Endodontics (ABE).
