CE Expiration Date: September 3, 2024
CEU (Continuing Education Unit):2 Credit(s)
AGD Code: 070
Educational aims and objectives
This self-instructional course for dentists aims to show information regarding endodontic treatment of the immature permanent necrotic teeth with open apices.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Realize why traditional root canal treatment may not be possible because of trauma or infection.
- Realize some background of regenerative endodontic therapy (RET).
- Identify some disadvantages of calcium hydroxide apexification.
- Identify some advantages of MTA apexification.
- Identify some disadvantages of MTA and bioceramic apexification.
- Realize some science related to platelet-rich fibrin (PRF).
- Observe a case report reflecting this technique.
Drs. Tony Tataro and Mohammad Sabeti show how an MTA apical plug can be effective, biocompatible, and have great sealing ability.
Drs. Tony Tataro and Mohammad Sabeti discuss and illustrate an alternative treatment
Introduction
The Hertwig epithelial root sheath (HERS) and the apical papilla interact epithelially and mesenchymally to mediate the root formation process.1 As stem cells move and develop into odontoblasts and fibroblasts, the pulp-dentin complex is generated at the expense of the apical papilla, lowering the apical papilla size as the root matures. Unfortunate events such as trauma and infection can impede this sophisticated growth process and interfere with normal root development, resulting in insufficient root apex formation.2,3 Traditional root canal treatment is not a good option in these situations since dentin formation ceases when pulp vitality is lost, resulting in a tooth with open apices and thin dentin walls that is prone to fracture.
An apical plug has typically been used to treat immature permanent necrotic teeth with open apices.4,5 This technique involves either the application of long-term calcium hydroxide or immediate placement of a mineral trioxide aggregate (MTA) plug.
Despite the ability to provide a seal and allow obturation, these techniques do not result in increased root length or width development.6 An alternative treatment option for immature permanent teeth with open apices is regenerative endodontic therapy (RET). Dr. Nygaard-Ostby’s study paved the way for regenerative endodontics.7 He speculated that a blood clot could be the first stage in the healing of a damaged dental pulp, comparable to the role of blood clots in other healing processes (e.g., alveolar bone recovery after extractions).7 This strategy is based on the idea that induction of a blood clot will stimulate the pulpal stem cell to regenerate the pulp-dentin complex and enhance the development and maturation of the root.8 The goal of this treatment alternative is not only to result in healing of any apical lesions, but also to gain root dentin thickness and restore the pulp dentin complex.
Apexification
Calcium hydroxide apexification
Apexification has traditionally been done with calcium hydroxide. This is a complex operation that takes from 5 to 20 months to complete and requires several appointments.3 The tooth is temporarily restored during this time and is vulnerable to fracture. In addition, after being exposed to calcium hydroxide for 5 weeks or longer, multiple in vitro investigations found that the mechanical characteristics of radicular dentin may be reduced.9
Disadvantages of calcium hydroxide apexification
- The time required for formation of the calcified barrier (3 to 24 months).
- Multiple appointments needed for reapplication of calcium hydroxide.
- The effect of long-term (several months or more) calcium hydroxide on the mechanical properties of dentin, including increase risk of root fracture.10
- Formation of a “swiss-cheese-like” apical barrier (which involves soft tissue inclusions inside the hard tissue) that can lead to poorer seal.
MTA apexification
MTA (mineral trioxide aggregate) was first introduced in 1993 and has been widely researched since then. It is a great alternative to calcium hydroxide as an apical barrier in immature, nonvital teeth. MTA is a bioactive material that has great biocompatibility, sealing capabilities, and antibacterial characteristics.11,12
Advantages of MTA apexification
- Consistent, more predictable apical barrier formation13
- Fewer appointments to complete the treatment
- Reduced follow-up appointments
- Higher success rate (95%)14
MTA and bioceramic apexification disadvantages
MTA has some drawbacks, including a long setting time, high cost, difficult handling properties, and dentin discoloration.11,12 Other bioactive materials that can be employed as apical barriers have been developed. These bioceramics include EndoSequence® Root Repair Material (ERRM) putty (Brasseler) and Biodentine® (Septanest® N, Septodont). The majority of articles describe beneficial features similar to MTA such as biocompatibility, bioactivity, limited microleakage, and a low toxicity.15,16 In addition to these features, some added benefits have been described for these new bioceramics, including improved clinical handling capabilities and no staining.
MTA plug and extraradicular matrix
Biological consequences
The use of an extraradicular matrix for the treatment of immature teeth with open apices is justified by a number of challenges encountered in these cases. First, the canal is generally broader apically than coronally and thus a filling method, including softened filling material, is necessary, so it can mold and adapt to the shape of the apical part of the canal. There is no barrier to prevent this softened material from extruding from the apex leading to possible inflammatory reactions. Furthermore, the lack of an apical stop due to material extrusion rather than plug formation may result in an unsealed canal that is vulnerable to leakage.17
Another challenge is that these immature teeth have thin radicular dentin and are more prone to fracture during treatment. These issues are addressed by promoting the creation of a hard-tissue barrier to allow for efficient canal filling and by strengthening the weaker root against fracture during and after apexification.16,18,19 In addition, platelet-rich fibrin (PRF) releases the chemical and cellular components required for healing. The use of a biocompatible matrix is said to give the delivery of the apical plug better control. Several matrix materials such as demineralized freeze-dried bone, collagen sponges, calcium sulfate, and PRF have been suggested.
The science of platelet-rich fibrin (PRF)
A popular extracellular matrix material is PRF. Many of the chemical and cellular components required for healing are found in whole blood. The main components of whole blood include plasma and three main types of cells: red blood cells, white blood cells, and platelets. Platelets recruit leukocytes and play an important role in innate immunity. These activities aid in tissue regeneration and repair.
Technique
A 10 mL sterile glass tube with no additives is used to draw venous blood.
The blood should be centrifuged as soon as possible at 2700 rpm for 12 minutes. The blood will separate into three layers: red blood cells on the bottom, platelet-poor plasma (PPP) on top, and PRF fibrin clot in the middle (Figure 1). This high-density fibrin clot acts as a biological matrix, allowing cells to migrate and produce cytokines. PRF leukocytes operate as anti-inflammatory agents and play an important part in immunological control.20 The PRF clot can be compressed into a membrane and utilized whole, or it can be split into smaller pieces and used separately (Figure 2).

Clinical technique of using PRF as an apical barrier
An apical plug can be used as a barrier outside the root, and the whole root is filled with MTA or any bioceramic materials (Figure 3).
First appointment
- Review the patient medical history and dental data and baseline
- Obtain anesthesia, and place rubber dam to isolate the tooth.
- Once the tooth is accessible, determine working length.
- After access, clean and shape the canal.
- Irrigate the root canal system first with 1.5 NaOCl (20 ml/canal for 5 min) and then irrigate with saline.21
- Slowly inject calcium hydroxide into the canal space to the working length.
- Place the microsponges and temporary restoration consisting GC Fuji TRIAGE® in the access.
Second appointment after 4 weeks
Perform clinical exam to ensure that there are no signs or symptoms (moderate-to-severe pain, pain on percussion, palpation, sinus tract, or swelling). If the patient presents with any symptoms or signs, repeat first appointment treatment.22 If not, proceed with the following steps:
- Determine tooth shade by using the vita classical A1-D4 shade guide.
- Obtain anesthesia with 2% lidocaine with 1:100,000 epinephrine.
- Place rubber dam to isolate the tooth.
- Re-access and remove the calcium hydroxide with 17% EDTA (20 ml/canal for 5 min).
- Perform a final flush with saline, and dry the canal with sterile paper points.
- Draw blood using a 10-ml vacutainer without any additives and perform the following steps:
- Centrifuge at 2700 rpm for 12 minutes. The blood will separate into platelet-poor plasma, a PRF fibrin clot, and RBCs.
- Separate the fibrin clot by pulling in with tweezers and cut as
- Use a PRF box to compress the PRF clot.
- Cut the compressed PRF membrane to size, and insert into the root canal to the apex of the tooth.
- Place multiple pieces until a firm apical barrier is formed.
- Fill the canal with the MTA to the cementoenamel junction or 3 mm -5 mm from the radiographic apex, and obturate with gutta percha to the cementoenamel junction (CEJ).
- Place the composite restoration over the MTA.
Case report
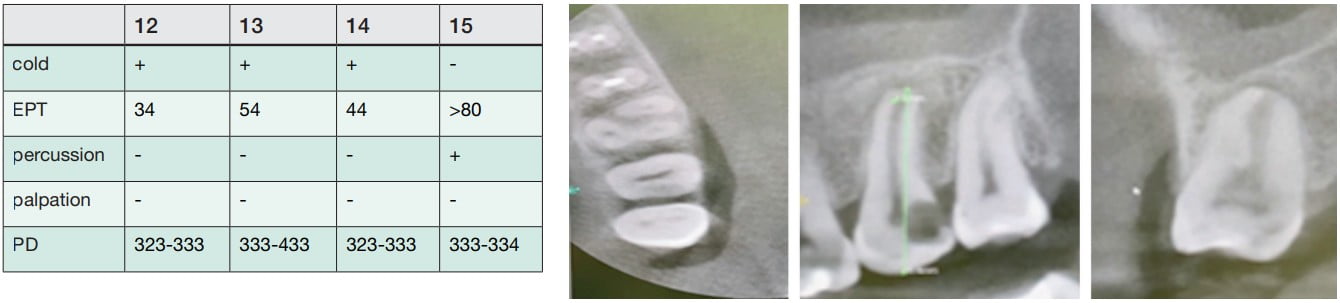
A 43-year-old female was referred from the predoctoral clinic. Her medical history was noncontributory. She said her chief complaint was “I had severe pain on upper left side,” pointing at tooth No. 15.
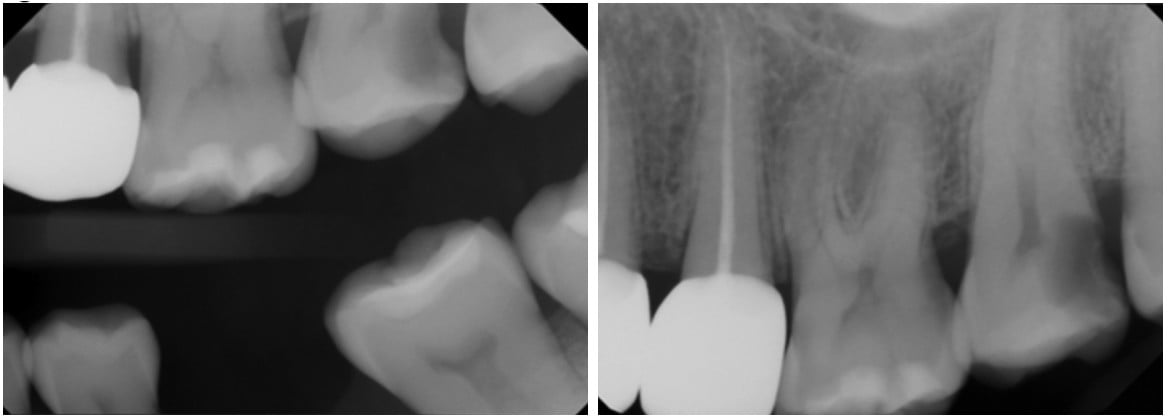
Clinical and radiographical examination reveal that tooth No. 15 is necrotic with symptomatic apical periodontitis and has decay on the distal surface (Figures 4 and 5). Cone beam computed tomography indicates that tooth No. 15 has a periapical radiolucency and a mesiobuccal (MB) canal that is joining the palatal canal (Figure 6). After cleaning and shaping of the canals, the MB was obturated with gutta percha (Figure 7). The palatal canal had an open apex, and thus it was decided to use MTA to create an apical plug. The remainder of the canal was obturated with gutta percha (Figure 10).
First Appointment

Second Appointment
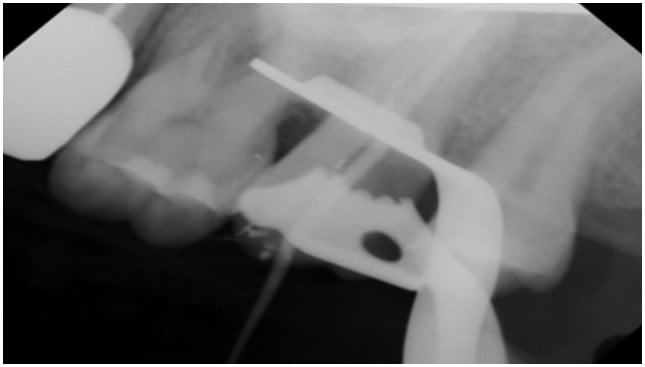
MTA Plug

Conclusion
Necrotic immature permanent teeth can be managed by using an MTA apical plug. An MTA apical plug encourages the formation of a calcified barrier at the end of a root canal, which facilitates the vertical condensation of warm gutta percha. An MTA plug is effective and biocompatible, has great sealing ability, and can be performed in one single visit.
An MTA apical plug was the treatment of choice in this article by Dr. Jorge Alberdi. Read about it here: https://endopracticeus.com/apexogenesis-and-apexification-with-mineral-trioxide-aggregate-mta-a-report-of-two-cases/
References
- Xu L, Tang L, Jin F, Liu X-H, et al. The apical region of developing tooth root constitutes a complex and maintains the ability to generate root and periodontium-like tissues. J Periodontal Res. 2009;44(2):275-282.
- Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8(2):45-55.
- Sheehy EC, Roberts GJ. Use of calcium hydroxide for apical barrier formation and healing in non-vital immature permanent teeth: a review. Br Dent J. 1997;183(7):241-246.
- Witherspoon DE, Small JC, Regan JD, Nunn M. Retrospective analysis of open apex teeth obturated with mineral trioxide aggregate. J Endod. 2008;34(10):1171-1176.
- Cvek M. Treatment of non-vital permanent incisors with calcium hydroxide. IV. Periodontal healing and closure of the root canal in the coronal fragment of teeth with intra-alveolar fracture and vital apical fragment. A follow-up. Odontol Revy. 1974;25(3):239-246.
- Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35(10):1343-1349.
- Nygaard-Ostby B. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol Scand. 1961;19:324-353.
- Musson DS, McLachlan JL, Sloan AJ, Smith AJ, Cooper PR. Adrenomedullin is expressed during rodent dental tissue development and promotes cell growth and mineralization. Biol Cell. 2010;102(3):145-157.
- Yassen GH, Platt JA. The effect of nonsetting calcium hydroxide on root fracture and mechanical properties of radicular dentine: a systematic review. Int Endod J. 2013;46(2):112-118.
- Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18(3):134-137.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–Part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16-27.
- Shabahang S, Torabinejad M, Boyne PP, Abedi H, McMillan P. A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25(1):1-5.
- Sarris S, Tahmassebi JF, Duggal MS, Cross IA. A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children-a pilot study. Dent Traumatol. 2008;24(1):79-85.
- Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod. 2011;37(6):793-798.
- Leal F, De-Deus G, Brandão C, Luna A, Souza E, Fidel S. Similar sealability between bioceramic putty ready-to-use repair cement and white MTA. Braz Dent J. 2013;24(4):362-366.
- Kerekes K, Heide S, Jacobsen I. Follow-up examination of endodontic treatment in traumatized juvenile incisors. J Endod. 1980;6(9):744-748.
- Alhadainy HA, Abdalla AI. Artificial floor technique used for the repair of furcation perforations: a microleakage study. J Endod. 1998;24(1):33-35.
- Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21(3):109-112.
- Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, Landesberg R. Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg. 2005;63(4):521-528.
- Cvek M, Nord CE, Hollender L. Antimicrobial effect of root canal débridement in teeth with immature root. A clinical and microbiologic study. Odontol Revy. 1976;27(1):1-10.
- Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24(3):119-125.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


