CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
Part 2 of this article aims to show the reader how to improve the prognosis of root-canal-treated teeth by sealing the canal and minimizing the leakage of oral fluids and bacteria into the periradicular areas.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz online at endopracticeus.com to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Realize the important role that sealers may play by minimizing coronal leakage.
- Recognize some considerations regarding the temporary restoration’s ability to prevent coronal leakage and how the material behaves under functional loading and thermocycling.
- Recognize the role the smear layer can play to prevent sealer penetration into the dentinal tubules.
- Recognize that irrigation is key to removal of the smear layer lining the canal walls.
- Realize that the sealer used is as important as the core material that is placed within the canal.
Dr. Gregori Kurtzman continues his discussion of treatment and management of coronal leakage.
Dr. Gregori M. Kurtzman continues his discussion of coronal leakage prevention
Introduction
As discussed in part 1, coronal leakage is a frequent cause of endodontic failure related to salivary bacteria percolating between the coronal aspect of the tooth, down the obturated canals to lead to apical reinfection. This can be prevented by how the tooth is treated immediately before initiation of endodontic treatment through that care, and how the tooth is managed before the definitive restorative phase can be initiated.
Coronal restoration (access sealing)
As microorganisms have been shown to be able to penetrate through different temporary restorative materials and a supposedly well-obturated root canal system, the use of adhesive sealers may play an important role by minimizing coronal leakage. In addition, the importance of an immediate definitive coronal seal should be emphasized after obturation of the canal system.1-3
One study reported that 70 extracted single-rooted mandibular premolars were studied to determine the length of time needed for bacteria present in saliva to penetrate through three commonly used temporary restorative materials and through the entire root canal system obturated with the lateral condensation technique. The average time observed for contamination of access cavities sealed with gutta percha (7.85 days), IRM (12.95 days), and Cavit-G (9.80 days). Thus, indicating that even during short periods of time normally permitted between visits, complete leakage may result. IRM, a common temporary material, was shown to leak to a significantly higher degree then glass ionomers.4,5 Glass-ionomer cement due to its adhesive nature has demonstrated an ability to prevent bacterial penetration to the periapex of obturated teeth for over a 1-month period as compared to IRM or Cavit™ (3M ESPE) temporary restorations.6
Another important consideration, regarding the temporary restoration’s ability to prevent coronal leakage, is how the material behaves under functional loading and thermocycling.7 Nonadhesive temporaries present with a greater degree of marginal breakdown and increased microleakage after thermocycling and loading. There was no significant improvement with increased thickness of the temporary material.8-10 When teeth were sealed with IRM, recontamination was detected within 13.5 days in the canals medicated with chlorhexidine, after 17.2 days in the group medicated with Ca(OH)2 and after 11.9 days in the group medicated with both chlorhexidine and Ca(OH)2. The group that had no medication but was sealed with IRM demonstrated recontamination after 8.7 days. Statistically significant differences between the teeth with or without coronal seal were observed. A coronal seal delayed but did not prevent leakage of microorganisms.11 This has been confirmed in other studies that IRM started to leak after 10 days, whereas Cavit and Dyract® (Dentsply) leaked after 2 weeks.12,13
Utilization of a resin-based temporary restorative material or glass ionomer over partially removed resin composite restorations could be beneficial in achieving better resistance to marginal leakage (Figure 1). Maintaining partially removed permanent restorations does not seem to cause a problem with achieving marginal seal.14 Glass ionomers demonstrated a statistically better coronal seal then bonded composite or even a bonded amalgam preventing bacterial apical migration.15 This appears to be related to the glass ionomer’s ability to adhere to sclerotic dentin found on the pulpal floor better then adhesive resins.16 Key to healing of periapical lesions following completion of endodontic treatment seems to be locking out coronal bacteria, and the apical area will heal (Figures 2 and 3).

Mineral trioxide aggregate (MTA) has since its introduction been advocated as a sealing material especially when perforation has occurred. But an investigation found mild inflammation was observed in 17% and 39% of the roots with and without an orifice plug, respectively; without development of severe inflammation, the sealing efficacy of MTA orifice plugs could not be determined.17,18
Should amalgam be the material of choice for the dentist, a bonded amalgam produced significantly less leakage than did the non-bonded amalgams. To prevent the reinfection of the endodontically treated molar, it may be preferable to restore the tooth immediately after obturation by employing a bonded amalgam coronal-radicular technique.19 Whereas, good long-term leakage resistance with a core buildup or access closure, with adhesive materials has been shown. A GI base with overlaying composite (referred to as the “sandwich” technique) or a composite resin restoration allowed significantly less coronal leakage than glass ionomer cement restorations. This may be because the composite resin prevents salivary dissolution of the glass ionomer long term.20
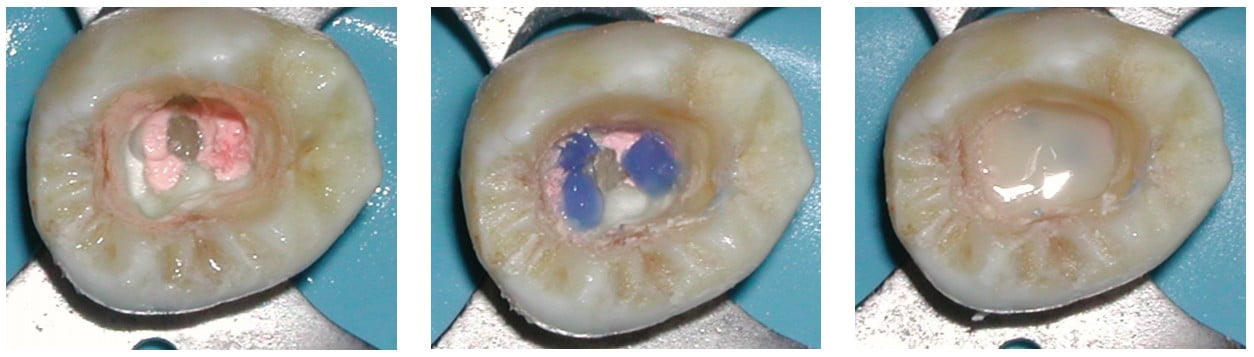
Results indicate that the sealing ability of adhesive and flowable materials can decrease coronal leakage potential.21 Because of the risk of coronal microleakage, endodontically treated teeth should be restored as quickly as possible.22 It is more prudent to use a permanent restorative material for provisional restorations to prevent potential for coronal leakage and the resulting risk of bacterial penetration through the canal system between endodontic treatment appoointments.23 To minimize the potential of perforation when re-entering the tooth to place either a post at a subsequent appointment or for endodontic retreatment should that be necessary at a later date, placement of a contrasting colored resin over each orifice may be beneficial. This is followed by covering the entire pulpal floor with a tooth-colored flowable resin (Figures 4-6). These are available in a multitude of easily identifiable colored flowable composites available in pink (PermaFlo® Pink) or purple (PermaFlo® Purple) from Ultradent (South Jordan, UT) or dark blue from DenMat (Santa Maria, CA).
Coronal microleakage has received considerable attention as a factor related to failure of endodontic treatment, and much emphasis is placed on the quality of the final restoration. Intracanal posts are frequently used for the retention of coronal restorations. Many authors have examined coronal microleakage with respect to gutta-percha root fillings and coronal restorations, but few have investigated the coronal seal afforded by various post systems. The seal provided by a cemented post depends on the seal of the cement used. It appears that the dentin-bonding cements (adhesive resins and glass ionomers) have less microleakage than the traditional, non-dentin-bonding cements (i.e., zinc phosphates and polycarboxolates).24 Resin fiber and glass fiber posts showed lower coronal leakage when compared with metal (stainless steel or titanium) and zirconia posts. This may be related to superior adhesion of the luting agent to these resin impregnated posts than to metal or ceramic posts, which do not allow adhesive penetration to the surface of the post. There were no significant differences between resin fiber and glass fiber posts at any time period. The initial leakage measurement in zirconia and metal posts were similar but became significantly different at 3 and 6 months. Those resin fiber and glass fiber posts tested exhibited less microleakage compared to zirconia post systems.25
Cleansing the canal (smear layers)
Coronal sealing ability is not the only factor that influences the seal of the canal and prevents apical leakage. How well the sealer adheres to the canal walls is also important. Smear layer can play a factor, which may prevent sealer penetration into the dentinal tubules. The frequency of bacterial penetration through teeth obturated with intact smear layer (70%) was significantly greater than that of teeth from which the smear layer had been removed (30%). Smear layer removal enhanced sealability as evidenced by increased resistance to bacterial penetration.26 Apical leakage incidence was reduced in the absence of the smear, and the adaptation of gutta percha was improved no matter what obturation method was used later.27-29 However, regardless of the obturation technique (single cone, lateral, vertical condensation, or thermoplastized), when a nonadhesive sealer was utilized, leakage increased after 30 days.30
What material used for obturation of the canals is important; however, the manner in which the canal was prepared prior to obturation also determines how well the canal is sealed when treatment is completed. Rotary instrumentation with NiTi files has demonstrated less microleakage than hand instrument prepared canals irrespective of what was used to obturate the canal.31 The better the canal walls are prepared, the more smear layer and organic debris is removed, which is beneficial to root canal sealing. NiTi rotary instrument machining of the canal walls provides a smoother canal wall and shapes that are easier to obturate than can be achievable with hand files. The resulting better adaptation of obturation material to the instrumented dentinal walls, the less leakage is to be expected along the entire root length.
Smear layer removal is best achieved by irrigating the canals with sodium hypochlorite (NaOCL) followed by 17% EDTA solution,32,33 whereas the NaOCL dissolves the organic component of the smear layer exposing the dentinal tubules lining the canal walls. EDTA, a chelating agent, dissolves the inorganic portion of the dentin opening the dentinal tubules. Alternating between the two irrigants as the instrumentation is being performed will permit removal of more organic debris further into the tubules, increasing resistance to bacterial penetration once the canal is obturated.34-36
Obturation
The purpose of the obturation phase of endodontic treatment is twofold: to prevent microorganisms from re-entering the root canal system and to isolate any micro-organisms that may remain within the canal system from nutrients in oral or tissue fluids. No matter how well we seal the canal, if the coronal portion of the tooth is not thoroughly sealed, then bacterial leakage may just be a matter of time. Accessory canals maybe present in the pulp chamber leading to the furcation area, which may be an additional source of leakage that often goes unaddressed either following obturation of the canal system or during the restorative phase. Placement of a layer of resin-modified glass ionomer cement or an adhesive resin to seal this area immediately following obturation can prevent leakage prior to final restoration of the tooth.37 But success can only be achieved if the root canal system has been as thoroughly debrided as possible of pulpal tissue and the bacteria associated with it lining the canal system walls (smear layer). To this goal, irrigation is key to removal of this smear layer lining the canal walls.
The obturation material is a double-edged sword. Which sealer is used is as important as which core material is placed within the canal. Gutta percha has limitations in resistance to coronal leakage which have been overcome with the newer resin alternatives. Although sealers can form close adhesion to the root canal wall, none is able to bond to the gutta-percha core material. Upon setting, shrinkage of the sealer allows the sealer to pull away from the gutta-percha core, leaving a microgap gap through which bacteria may pass.38 Several alternatives are available for core material selection.
Gutta percha demonstrates leakage in 80% of specimens related to coronal leakage when inadequate coronal sealing is not achieved, which is not dependent on obturation technique nor which sealer was used.39 Because of these limitations seen with gutta percha, the seal of a coronal restoration may be as important as the gutta-percha fill-in preventing reinfection of the root canal.40 The significance of this is, should the coronal break down, the adhesive obturation material may slow down or prevent apical migration of bacteria allowing healing to occur (Figures 7 and 8).

Sealer selection is very important in prevention of microleakage and permits a bond to the core material. Zinc oxide and eugenol (ZOE) sealers have been a mainstay in endodontic therapy for over 100 years. When exposed to coronal leakage, ZOE sealers demonstrated complete leakage by the second day. Results indicated that none of the ZOE formulations tested could predictably produce a fluid-tight seal even up to the 4th day.41
AH-26® (Dentsply), an epoxy sealer originally introduced over 40 years ago, was also unable to bond to gutta percha, leading to coronal leakage issues. Leakage with AH-26 was not dependent on obturation technique showing gross leakage increasing within the first 4 months following obturation when coronally challenged. Coronal leakage was significantly greater during the first 4 months.42 Complete bacterial leakage with AH-26 may be seen in as few as 8.5 weeks should the coronal restoration permit leakage.43 Additionally, in vitro studies found gutta percha and AH-26 or AH-26 Plus permitted leakage of both bacteria and fungi. Leakage in experimental teeth occurred between 14 and 87 days with 47% of the samples showing leakage. AH-26 sealer permitted bacterial leakage in 45% and fungi leakage in 60% samples. Whereas the samples with AH Plus demonstrated bacterial leakage in 50% and fungi 55% of the samples. There was no statistically significant difference in penetration of bacteria and fungi between the two versions of the sealer.44 As AH-26 is unable to bond to gutta percha, polymerization shrinkage of the epoxy resin can result in a microgap leading to the leakage reported in the literature. (Figure 9) The goal is creation of a monoblock with no interspersed gaps between the canal wall, gutta percha (or alternative cone material), and sealer (Figure 10). Should the practitioner wish to continue using these materials, a permanent restoration needs to be placed at the appointment when endodontic therapy is completed.

Traditional sealers that have been in use in endodontics for many decades exhibit some cytotoxicity, especially if any extrudes apically during the obturation phase of treatment.45 These include calcium hydroxide Ca(OH)2 and zinc oxide eugenol (ZOE)-based sealers. An additional problem with these type sealers is when coronal leakage occurs, the sealer is prone to dissolution increasing leakage and the potential for endodontic failure. This has led to research to find alternative sealers with better properties that can resist coronal leakage and are more biocompatible.
Bioceramic sealers have been used increasingly in endodontics over the past 10 years. These materials are calcium silicate in chemistry. Studies have evaluated their physical properties, biocompatibility, sealing ability, adhesion, solubility, and antibacterial efficacy.46 These materials have been used in orthopedics for several decades, and biocompatibility has been verified with the material being non-host reactive following placement.47 The use as a replacement sealer in endodontic treatment was an extension of the success observed in orthopedics and its biocompatibility and ability to resist dissolution when challenged with fluids.48,49 Additionally, antimicrobial effects have been reported for various bioceramic sealers currently available for clinical use.50, 51 When compared to epoxy resin sealers (AH-26), calcium silicate sealers exerted higher antimicrobial effects against E faecalis biofilms for longer periods of time.52 These bioceramic sealers are provided as either ready-to-use sealers consisting of only one component (does not require mixing) with a need for external water supply from fluid in the canal system when obturation occurs and two component sealers with internal water supply that is mixed prior to use. Both of these material types have the similar setting reactions, whereby a hydration reaction of the calcium silicate is followed by a precipitation reaction of calcium phosphate.53 The result upon setting is a relatively insoluble sealer that can resist coronal leakage, thereby preventing reinfection of the canal system from salivary bacteria.
Conclusion
Of 41 articles published between 1969 and 1999 (the majority from the 1990s), the literature suggests that the prognosis of root-canal-treated teeth can be improved by sealing the canal and minimizing the leakage of oral fluids and bacteria into the periradicular areas as soon as possible after the completion of root canal therapy.54
Endodontic success is multifactorial. The full picture, like a jigsaw puzzle, can only be seen when all the pieces are fit together. How the canals are instrumented is as important as what is used to obturate the canal system. This is also influenced by what is placed coronally and when the coronal aspect is sealed. NiTi rotary instruments and an irrigation protocol that includes NaOCL and EDTA will maximize the sealing ability of glass ionomer or the newer methacrylic resin sealers. The last piece of the puzzle — sealing coronally — should be performed with adhesive permanent restorative materials immediately at the conclusion of the first endodontic appointment to prevent apical migration of bacteria and assure sealability of the canals.
Read part one of Dr. Kurtzman’s CE, “Improving endodontic success through coronal leakage prevention” here: https://endopracticeus.com/ce-articles/improving-endodontic-success-through-coronal-leakage-prevention-part-1/
References
- Imura N, Otani SM, Campos MJA, Jardim EG, Zuolo ML. Bacterial penetration through temporary restorative materials in root-canal-treated teeth in vitro. Inter Endod J. 1997;30:381-385
- Uranga A, Blum JY, Esber S, Parahy E, Prado C.: A comparative study of four coronal obturation materials in endodontic treatment. J Endod. 1999;25(3):178-180.
- Fox K, Gutteridge DL.: An in vitro study of coronal microleakage in root-canal- treated teeth restored by the post and core technique. Int Endod J. 1997;30(6):361-368
- Barthel CR, Zimmer S, Wussogk R, Roulet JF.: Long-Term bacterial leakage along obturated roots restored with temporary and adhesive fillings. J Endod. 2001;27(9):559-562
- Babu NSV, Bhanushali PV, Bhanushali NV, Patel P. Comparative analysis of microleakage of temporary filling materials used for multivisit endodontic treatment sessions in primary teeth: an in vitro study. Eur Arch Paediatr Dent. 2019;20(6):565-570.
- Barthel CR, Strobach A, Briedigkeit H, Gobel UB, Roulet JF. Leakage in roots coronally sealed with different temporary fillings. J Endod. 1999;25(11):731-734
- Balkaya H, Topçuoğlu HS, Demirbuga S. The Effect of Different Cavity Designs and Temporary Filling Materials on the Fracture Resistance of Upper Premolars. J Endod. 2019;45(5):628-633.
- Mayer T, Eickholz P. Microleakage of temporary restorations after thermocycling and mechanical loading. J Endod. 1997;23(5):320-322
- Deveaux E, Hildelbert P, Neut C, Boniface B, Romond C. Bacterial microleakage of Cavit, IRM, and TERM. Oral Surg Oral Med Oral Pathol. 1992;74(5):634-643
- Deveaux E, Hildelbert P, Neut C, Romond C. Bacterial microleakage of Cavit, IRM, TERM, and Fermit: a 21-day in vitro study. J Endod. 1999;25(10):653-659
- Gomes BP, Sato E, Ferraz CC, Teixeira FB, Zaia AA, Souza-Filho FJ. Evaluation of time required for recontamination of coronally sealed canals medicated with calcium hydroxide and chlorhexidine. Int Endod J. 2003 Sep;36(9):604-609.
- Balto H.: An assessment of microbial coronal leakage of temporary filling materials in endodontically treated teeth. J Endod. 2002;28(11):762-764.
- Balto H, Al-Nazhan S, Al-Mansour K, Al-Otaibi M, Siddiqu Y. Microbial leakage of Cavit, IRM, and Temp Bond in post-prepared root canals using two methods of gutta-percha removal: an in vitro study. J Contemp Dent Pract. 2005;6(3):53-61.
- Tulunoglu O, Uctasli MB, Ozdemir S.: Coronal microleakage of temporary restorations in previously restored teeth with amalgam and composite. Oper Dent. 2005;30(3):331-7.
- Nup C, Boylan R, Bhagat R, Ippolito G, Ahn SH, Erakin C, Rosenberg PA. An evaluation of resin-ionomers to prevent coronal microleakage in endodontically treated teeth. J Clin Dent. 2000;11(1):16-19.
- Karakaya S, Unlu N, Say EC, Ozer F, Soyman M, Tagami J. Bond strengths of three different dentin adhesive systems to sclerotic dentin. Dent Mater J. 2008;27(3):471-479.
- Mah T, Basrani B, Santos JM, Pascon EA, Tjaderhane L, Yared G, Lawrence HP, Friedman S.: Periapical inflammation affecting coronally-inoculated dog teeth with root fillings augmented by white MTA orifice plugs. J Endod. 2003;29(7):442-446.
- Alves AMH, Pozzobon MH, Bortoluzzi EA, et al. Bacterial penetration into filled root canals exposed to different pressures and to the oral environment-in vivo analysis. Clin Oral Investig. 2018;22(3):1157-1165.
- Howdle MD, Fox K, Youngson CC.: An in vitro study of coronal microleakage around bonded amalgam coronal-radicular cores in endodontically treated molar teeth. Quintessence Int. 2002;33(1):22-29.
- Kleitches AJ, Lemon RR, Jeansonne BG. Coronal microleakage in conservatively restored endodontic access preparations. J Tenn Dent Assoc. 1995;75(1):31-34.
- Shindo K, Kakuma Y, Ishikawa H, Kobayashi C, Suda H. The influence of orifice sealing with various filling materials on coronal leakage. Dent Mater J. 2004;23(3):419-423.
- de Souza FD, Pecora JD, Silva RG. The effect on coronal leakage of liquid adhesive application over root fillings after smear layer removal with EDTA or Er:YAG laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(1):125-128.
- Uranga A, Blum JY, Esber S, Parahy E, Prado C. A comparative study of four coronal obturation materials in endodontic treatment. J Endod. 1999;25(3):178-180.
- Ravanshad S, Ghoreeshi N. An in vitro study of coronal microleakage in endodontically-treated teeth restored with posts. Aust Endod J. 2003;29(3):128-133.
- Usumez A, Cobankara FK, Ozturk N, Eskitascioglu G, Belli S. Microleakage of endodontically treated teeth with different dowel systems. J Prosthet Dent. 2004;92(2):163-169.
- Behrend GD, Cutler CW, Gutmann JL. An in-vitro study of smear layer removal and microbial leakage along root-canal fillings. Int Endod J. 1996;29(2):99-107
- Karagoz-Kucukay I, Bayirli G. An apical leakage study in the presence and absence of the smear layer. Int Endod J. 1994;27(2):87-93
- Saunders WP, Saunders EM.: Influence of smear layer on the coronal leakage of Thermafil and laterally condensed gutta-percha root fillings with a glass ionomer sealer. J Endod. 1994;20(4):155-158.
- Gencoglu N, Samani S, Gunday M. Dentinal wall adaptation of thermoplasticized gutta-percha in the absence or presence of smear layer: a scanning electron microscopic study. J Endod. 1993;19(11):558-562.
- Pommel L, Camps J. In vitro apical leakage of system B compared with other filling techniques. J Endod. 2001;27(7):449-451.
- von Fraunhofer JA, Fagundes DK, McDonald NJ, Dumsha TC. The effect of root canal preparation on microleakage within endodontically treated teeth: an in vitro study. Int Endod J. 2000;33(4):355-360.
- Morago A, Ruiz-Linares M, Ferrer-Luque CM, Baca P, Rodríguez Archilla A, Arias-Moliz MT. Dentine tubule disinfection by different irrigation protocols. Microsc Res Tech. 2019;82(5):558-563.
- Nogo-Živanović D, Kanjevac T, Bjelović L, Ristić V, Tanasković I. The effect of final irrigation with MTAD, QMix, and EDTA on smear layer removal and mineral content of root canal dentin. Microsc Res Tech. 2019;82(6):923-930.
- Clark-Holke D, Drake D, Walton R, Rivera E, Guthmiller JM. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J Dent. 2003;31(4):275-81.
- Vivacqua-Gomes N, Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. Influence of irrigants on the coronal microleakage of laterally condensed gutta-percha root fillings. Int Endod J. 2002;35(9):791-5.
- Zaparolli D, Saquy PC, Cruz-Filho AM. Effect of sodium hypochlorite and EDTA irrigation, individually and in alternation, on dentin microhardness at the furcation area of mandibular molars. Braz Dent J. 2012;23(6):654-658.
- Carrotte P.: Endodontics: Part 8. Filling the root canal system. Br Dent J. 2004;197(11):667-672.
- Teixeira FB, Teixeira EC, Thompson J, Leinfelder KF, Trope M.:Dentinal bonding reaches the root canal system. J Esthet Restor Dent. 2004;16(6):348-54.
- Maggio JD.: RealSeal–the real deal. Compend Contin Educ Dent. 2004;25(10A):834, 836.
- Shipper G, Trope M.: In vitro microbial leakage of endodontically treated teeth using new and standard obturation techniques. J Endod. 2004;30(3):154-158.
- Tewari S, Tewari S.: Assessment of coronal microleakage in intermediately restored endodontic access cavities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(6):716-9.
- De Moor RJ, Hommez GM.: The long-term sealing ability of an epoxy resin root canal sealer used with five gutta percha obturation techniques. Int Endod J. 2002;35(3):275-282.
- Chailertvanitkul P, Saunders WP, MacKenzie D, Weetman DA. An in vitro study of the coronal leakage of two root canal sealers using an obligate anaerobe microbial marker. Int Endod J. 1996;29(4):249-255.
- Miletic I, Prpic-Mehicic G, Marsan T, Tambic-Andrasevic A, Plesko S, Karlovic Z, Anic I. Bacterial and fungal microleakage of AH26 and AH Plus root canal sealers. Int Endod J. 2002;35(5):428-32.
- Fonseca DA, Paula AB, Marto CM, et al. Biocompatibility of Root Canal Sealers: A Systematic Review of In Vitro and In Vivo Studies. Materials (Basel). 2019;12(24):4113.
- Al-Haddad A, Che Ab Aziz ZA. Bioceramic-Based Root Canal Sealers: A Review. Int J Biomater. 2016;2016: 9753210.
- Oonishi H, Hench LL, Wilson J, et al. Comparative bone growth behavior in granules of bioceramic materials of various sizes. J Biomed Mater Res. 1999;44(1):31-43.
- Al-Haddad A, Che Ab Aziz ZA. Bioceramic-Based Root Canal Sealers: A Review. Int J Biomater. 2016;2016: 9753210.
- Jitaru S, Hodisan I, Timis L, Lucian A, Bud M. The use of bioceramics in endodontics – literature review. Clujul Med. 2016;89(4):470-473.
- Bukhari S, Karabucak B. The Antimicrobial Effect of Bioceramic Sealer on an 8-week Matured Enterococcus faecalis Biofilm Attached to Root Canal Dentinal Surface.J Endod. 2019;45(8):1047-1052.
- Du TF, Wu LD, Tang XZ, et al. Zhonghua Kou Qiang Yi Xue Za Zhi. 2019;54(10):656-661.
- Alsubait S, Albader S, Alajlan N, Alkhunaini N, Niazy A, Almahdy A. Comparison of the antibacterial activity of calcium silicate- and epoxy resin-based endodontic sealers against Enterococcus faecalis biofilms: a confocal laser-scanning microscopy analysis. 2019;107(4):513-520.
- Donnermeyer D, Bürklein S, Dammaschke T, Schäfer E. Endodontic sealers based on calcium silicates: a systematic review. 2019;107(4):421-436.
- Heling I, Gorfil C, Slutzky H, Kopolovic K, Zalkind M, Slutzky-Goldberg I. Endodontic failure caused by inadequate restorative procedures: review and treatment recommendations. J Prosthet Dent. 2002;87(6):674-678.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Dr. Gregori M. Kurtzman, DDS, MAGD, FAAIP, FPFA, FACD, FADI, DICOI, DADIA, is in private general dental practice in Silver Spring, Maryland. He is a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry and Endodontics and a former AAID Implant Maxi-Course assistant program director at Howard University College of Dentistry. Dr. Kurtzman has lectured internationally on the topics of restorative dentistry, endodontics and implant surgery and prosthetics, removable and fixed prosthetics, and periodontics. Dr. Kurtzman has published over 750 articles globally, several ebooks, and textbook chapters. He has earned Fellowship in the AGD, American College of Dentists (ACD), International Congress of Oral Implantology (ICOI), Pierre Fauchard, ADI, Mastership in the AGD and ICOI and Diplomat status in the ICOI, American Dental Implant Association (ADIA), and International Dental Implant Association (IDIA). Dr. Kurtzman is a consultant and evaluator for multiple dental companies. He has been honored to be included in the “Top Leaders in Continuing Education” by Dentistry Today annually since 2006 and was featured on their June 2012 cover. Dr. Kurtzman can be reached at: jdr_kurtzman@maryland-implants.com
Dr. Gregori M. Kurtzman, DDS, MAGD, FAAIP, FPFA, FACD, FADI, DICOI, DADIA, is in private general dental practice in Silver Spring, Maryland. He is a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry and Endodontics and a former AAID Implant Maxi-Course assistant program director at Howard University College of Dentistry. Dr. Kurtzman has lectured internationally on the topics of restorative dentistry, endodontics and implant surgery and prosthetics, removable and fixed prosthetics, and periodontics. Dr. Kurtzman has published over 750 articles globally, several ebooks, and textbook chapters. He has earned Fellowship in the AGD, American College of Dentists (ACD), International Congress of Oral Implantology (ICOI), Pierre Fauchard, ADI, Mastership in the AGD and ICOI and Diplomat status in the ICOI, American Dental Implant Association (ADIA), and International Dental Implant Association (IDIA). Dr. Kurtzman is a consultant and evaluator for multiple dental companies. He has been honored to be included in the “Top Leaders in Continuing Education” by Dentistry Today annually since 2006 and was featured on their June 2012 cover. Dr. Kurtzman can be reached at: jdr_kurtzman@maryland-implants.com
