CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
This clinical article aims to discuss current approaches in the literature to furcation perforation and repair and regeneration in endodontology.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Take the quiz by clicking here. Correctly answering the questions will demonstrate the reader can:
- Identify surgical and non-surgical approaches that have been utilized for periodontal tissue re-establishment at the perforation site.
- Realize specific criteria for selecting repair material.
- Identify some essential prerequisites for the success of a perforation repair.
- Identify possible materials used for furcation perforation repair.
- Realize the essential role of cementum regeneration in the furcation perforation repair process.
Drs. Manal Farea, Adam Husein, and Cornelis H. Pameijer discuss current approaches and a new perspective for repair and regeneration in endodontology
During root canal treatment, many procedural accidents may occur of which perforation of the root canal system plays a significant role. Perforation is defined by the American Association of Endodontics (AAE) Glossary of Endodontic Terms (2003) as a mechanical or pathological communication between the root canal system and the external tooth surface, which is caused by caries, resorption, or iatrogenic factors. It has been identified as the second greatest cause of endodontic failure that accounts for 9.6% of all unsuccessful cases (Pitt Ford, et al., 1995).
As a result of furcation perforation, destruction of the periodontal tissues may occur, which ultimately lead to loss of the tooth (Arens, Torabinejad, 1996; Tsesis, Fuss, 2006). The prognosis of the tooth depends upon several factors:
- The severity of initial damage to the periodontal tissue
- The location and size of perforations
- The bacterial contamination
- The sealing ability or cytotoxicity of the repair materials (Tsesis, Fuss, 2006; Sinai, 1977; Balla, et al., 1991).
Even if a biocompatible material is used to treat a perforation, extensive injury may cause irreversible damage to the attachment apparatus at the furcation area (Sinai, et al., 1989.)
In large perforations, the complete sealing of the defect with a repair material is problematic and allows irritants to continuously penetrate into the furcation area (Balla, et al., 1991). Perforations close to the gingival sulcus produce persistent inflammation and a down-growth of sulcular epithelium into the defect (Tsesis, Fuss, 2006). Sinai (1977) stated that coronally located perforations, including furcal perforations, were more serious than those in the middle and apical third of a canal. It is the objective of this review to collect and review the data that is available in the scientific literature and to reach a conclusion as to the best treatment options.
Methods
Retrieval of literature
An English-limited Medline search was performed of articles published from 2002 to 2015. The searched keywords included perforations and endodontics, furcation perforation, root canal and perforation, and perforation and mineral trioxide aggregate (MTA). Then a hand search was done of the references of collected articles to determine if more papers relevant to the topic should be included.
Results
A total of 820 articles were found, which, in order of their related keywords, accounted for the following: perforations and endodontics: 285; furcation perforation: 92; root canal and perforation: 299; and perforation and mineral trioxide aggregate (MTA): 144.
Perforation repair techniques and their prognosis
Surgical and non-surgical approaches have been utilized for periodontal tissue re-establishment at the perforation site. In both surgical and non-surgical approaches, two factors should be considered:
- An appropriate material selection
- The use of a matrix (Clauder, Shin, 2009).
The repair material should be selected based on the following criteria:
- Perforation site accessibility
- Biocompatibility (be nontoxic and noncarcinogenic)
- Ability to induce osteogenesis and cementogenesis
- Moisture control
- Easy handling
- Esthetic considerations (Clauder, Shin, 2009; Bryan, Woollard, Mitchell, 1999; Yildirim, et al., 2005; Samiee, et al., 2010).
Matrix use
Controlling hemostasis and placement of the repair material in the perforation site without extrusion into surrounding periodontal structures are essential prerequisites for the success of a perforation repair. In order to achieve a fluid-tight seal, hemostasis has to be controlled (Clauder, Shin, 2009). Delayed perforation repair can lead to extrusion of repair materials as a result of breakdown of the surrounding periodontium that is replaced by granulation tissue. Thus, in an attempt to avoid extrusion of the repair material, internal matrices such as calcium sulfate, hydroxyapatite, collagen, demineralized freeze-dried bone, and Gelfoam® (Pfizer) have been used (Clauder, Shin, 2009; Roda, 2001; Bargholz, 2005).
The internal matrix concept was introduced by Lemon (1992) in order to adequately seal the furcation perforation and avoid extrusion of the material. He also recommended the use of hydroxyapatite as a matrix under amalgam.
Calcium sulfate and calcium hydroxide prevented extrusion of composite resin when used as a furcal repair material (Imura, et al., 1998). In 1999, Jantarat and colleagues demonstrated that amalgam placed with plaster of Paris as a matrix for furcal perforation repair improved its sealing ability. Hapset (65% non-resorbable hydroxyapatite and 35% plaster of Paris) and hydroxyapatite showed similar healing responses when used as internal matrices under amalgam (Rafter, et al., 2002). Rafter, et al. (2002), further reported that there was marked extrusion of amalgam into the underlying bone with an associated severe inflammatory response when used alone without a matrix.
Although it has been reported that without using an internal matrix the optimal strength and excellent sealability of MTA was achieved in the presence of moisture (Arens, Torabinejad, 1996; Holland, et al., 2001; Torabinejad, et al., 1994), conflicting results have been reported by some authors regarding the use of an internal matrix under MTA. In 2004, Kratchman suggested that the perforation site should be soaked with sodium hypochlorite after hemostasis had been achieved and that a physical barrier such as collagen or calcium sulfate must be used at the perforation site to prevent MTA from being packed into the bone.
According to Bargholz (2005), excellent clinical results were achieved when collagen matrix was used under MTA. A study by Al-Daafas and Al-Nazhan (2007) showed that calcium sulfate prevented extrusion of the repair material. However, an unfavorable inflammatory reaction — epithelial tissue migration into the defected perforation and the inability to induce bone regeneration — were detected.
Thus, the authors concluded that using calcium sulfate as an internal matrix for MTA is not recommended. When used as an internal matrix for furcal perforation repair, calcium sulfate, and Collaplug (Calcitek, Carlsbad, California) did not improve the sealing ability nor reduce the incidence of MTA overextension. Therefore, the authors concluded that these two materials are not recommended as an internal matrix for MTA (Zou, et al., 2008). Furthermore, calcium sulfate and hydroxyapatite did not improve the sealing ability of MTA when used as internal matrices for furcation perforation repair (Taneja, Kumari 2011).
Materials used for furcation perforation repair
In an attempt to repair a furcation perforation, several materials such as amalgam, tricalcium phosphate (TCP), hydroxyapatite, gutta percha, calcium hydroxide, zinc oxide-eugenol-based cement (IRM and Super-EBA), glass ionomer cement, composite resins, resin-glass ionomer hybrids, demineralized freeze-dried bone, and MTA have been used over the years (Arens, Torabinejad, 1996; Balla, et al., 1995; Bryan, Woollard, Mitchell, 1999, Yildirim, et al., 2005; Salman, et al., 1999). However, none fulfill all requisite qualifications for an ideal biomaterial.
Balla, et al. (1991), reported that no hard tissue was formed at the furcation perforation defect site when treated with either tri-calcium phosphate, hydroxyapatite, amalgam, or calcium hydroxide (Life™); instead, the defect site was occupied by epithelium and acute inflammatory cells (Balla, et al., 1991). MTA is water-based cement that is derived from Portland cement (Type I). It was introduced as a root-end filling material in the early 1990s (Torabinejad, Watson, Pitt Ford, 1993; Torabinejad, Chivian, 1999). It was subsequently determined that it was a suitable material for various clinical applications such as pulp capping, and repair of furcal perforations, as well as root-end closure (Sinai, et al., 1989; Torabinejad, et al., 1995). MTA promotes periradicular tissue regeneration (Pitt Ford, et al., 1995; Yildirim, et al., 2005; Holland, et al., 2001; Zhu, Xia, Xia, 2003; Noetzel et al., 2006), and it differs from other materials by its ability to promote cementum regeneration, thus facilitating the regeneration of the periodontal apparatus (Pitt Ford, et al., 1995; Arens, Torabinejad, 1996). Its biocompatibility nature is suggested by its ability to form hydroxyapatite when exposed to simulated body tissue fluid (Sarkar, et al., 2005).
Two commercial forms of MTA are available: ProRoot® MTA (Dentsply Tulsa Dental), which is available in both gray or white form, of which the latter contains a lower amount of iron, and MTA-Angelus (Angelus) (Asgary, et al., 2005). MTA-Angelus was introduced to address the long setting time from 2 hours for ProRoot MTA to 10 minutes for MTA-Angelus. MTA-Angelus contains 80% Portland cement and 20% bismuth oxide, with no addition of calcium sulfate, while ProRoot MTA is composed of 75% Portland cement, 20% bismuth oxide, and 5% calcium sulfate dehydrate (Hashem, et al., 2008). The constituents of the Portland cement are minerals, among which the most important are dicalcium silicate, tricalcium silicate, tricalcium aluminate, tetracalcium ironaluminate, and dehydrated calcium sulfate (Oliveira, et al., 2007; Asgary, et al., 2009). The only significant difference between the dominant compounds of white and gray MTAs and associated Portland cements is bismuth oxide, which is present in MTAs (Asgary, et al., 2009; Asgary, et al., 2004).
Figure 1: MTA Caps (Acteon)
Figure 2: ProRoot® MTA (Dentsply Tulsa Dental)
It has been reported that the sealing ability of MTA (Loma Linda University, Loma Linda, California) was significantly better compared to amalgam in preventing leakage of fusobacterium nucleatum through furcal perforations (Nakata, Bae, Baumgartner, 1998). When used to seal a large furcation perforation, ProRoot MTA with/without internal matrix and MTA-Angelus with internal matrix showed the lowest dye absorbance compared to zinc oxide-eugenol cement (IRM) with/without internal matrix and MTA-Angelus without internal matrix. Additionally, the authors reported that IRM without internal matrix had the highest dye absorbance (Hashem, Hassanien, 2008). However, white and gray MTA (Dentsply Tulsa Dental) showed no significant differences in microleakage when used for furcal perforation repair (Ferris, Baumgartner, 2004; Hamad, Tordik, McClanahan, 2006). Furcal perforations have been repaired with ProRoot gray MTA (Dentsply) and Geristore® (Denmat). Geristore has been used as a root end filling material and in the restoration of subgingival surface defects such as root surface caries and iatrogenic perforations, surgical repair of root perforations, and as an adjunct in guided-tissue regeneration (GTR) (Mehrvarzfar, et al., 2010). It also leaked significantly less than amalgam (Mehrvarzfar, et al., 2010). In the aforementioned study, the authors reported that the sealing ability of MTA and Geristore was reduced when bioglass was used as a matrix underneath.
Sluyk, Moon and Hartwell (1998) assessed the effect of time and moisture on setting, retention, and adaptability of MTA when used for furcal perforation repair. Findings showed that MTA adaptation to perforation walls increased in the presence of moisture. They further suggested that a moistened matrix can be used under MTA to prevent under- or overfilling of the material. Furthermore, Main, et al. (2004), indicated that MTA provided an effective seal for root perforations.
Yildirim, et al. (2005), investigated the histologic response to MTA and Super EBA (Bosworth Company) when used in furcation perforation repair in dogs. In their study, less inflammation and new cementum formation was observed with MTA compared to Super EBA, which demonstrated connective tissue repair without inflammation. Similar abilities to seal furcal perforations were observed for both Portland cement and MTA (De-Deus, et al., 2006; Noetzel et al, 2006) evaluated histologically the inflammatory reactions and tissue responses to experimental tricalcium phosphate (TCP) and MTA when used as repair materials in furcation perforations in dogs. Results showed no significant differences between MTA and TCP in terms of bone reorganization or deposition of fibrous connective tissue.
Thus, MTA is considered the gold standard and material of choice for perforation repair and has demonstrated good potential for clinical success. However, it has some disadvantages, including the inability to degrade to allow for replacement with natural tissues, low resistance to compression over the long-term, extended setting time, poor handling, and difficult insertion into cavities because of its granular consistency, while additional moisture is required to activate the cement setting, and lastly, the high cost, despite its widespread use (Torabinejad, et al., 1995; Chng, et al., 2005; Kogan, et al., 2006; Coomaraswamy, Lumley, Hofmann, 2007; Parirokh, Torabinejad, 2010).
Many dental materials have been demonstrated in the literature to exhibit cytotoxic effects during setting. Low cell numbers were demonstrated in vivo with freshly mixed MTA (pH=10.2) compared to preset MTA (pH=12.5) (Tronstad, Wennberg, 1980). However, histologically, no difference in bone and cementum regeneration was observed after periradicular surgery in dogs between fresh and preset ProRoot MTA (Apaydin, Shabahang, Torabinejad, 2004).
In 2006, Asgary and colleagues introduced a new endodontic cement, a calcium-enriched mixture (CEM) cement. Major components of CEM cement powder are 51.75 wt.% calcium oxide, 9.53 wt.% sulfur trioxide, 8.49 wt.% phosphorous pentoxide, and 6.32 wt.% silicon dioxide; whereas the minor essential constituents are aluminium oxide > sodium oxide > magnesium oxide > chlorine. CEM cement has a similar pH but an increased flow compared to MTA. However, working time, film thickness, and price are considerably less (Asgary, et al., 2008a). Unlike MTA, mixed CEM cement releases calcium and phosphate ions and forms hydroxyapatite not only in simulated body tissue fluid but also in normal saline solution (Asgary, et al., 2009; Amini, et al., 2009).
Although the chemical composition of CEM cement and MTA are different, they have similar clinical applications (Asgary, et al., 2008; Asgary, et al., 2008; Asgary, et al., 2009; Asgary, Ehsani, 2009). Similar to MTA, CEM cement had low cytotoxic effects on different cell lines (Asgary, et al., 2009). However, it showed a better antibacterial effect comparable to calcium hydroxide (Asgary, et al., 2008). Similar sealing ability was demonstrated by both ProRoot MTA and CEM when used to repair furcal perforation of primary molar teeth (Haghgoo, et al., 2014).

Figure 3: Harvard MTA OptiCaps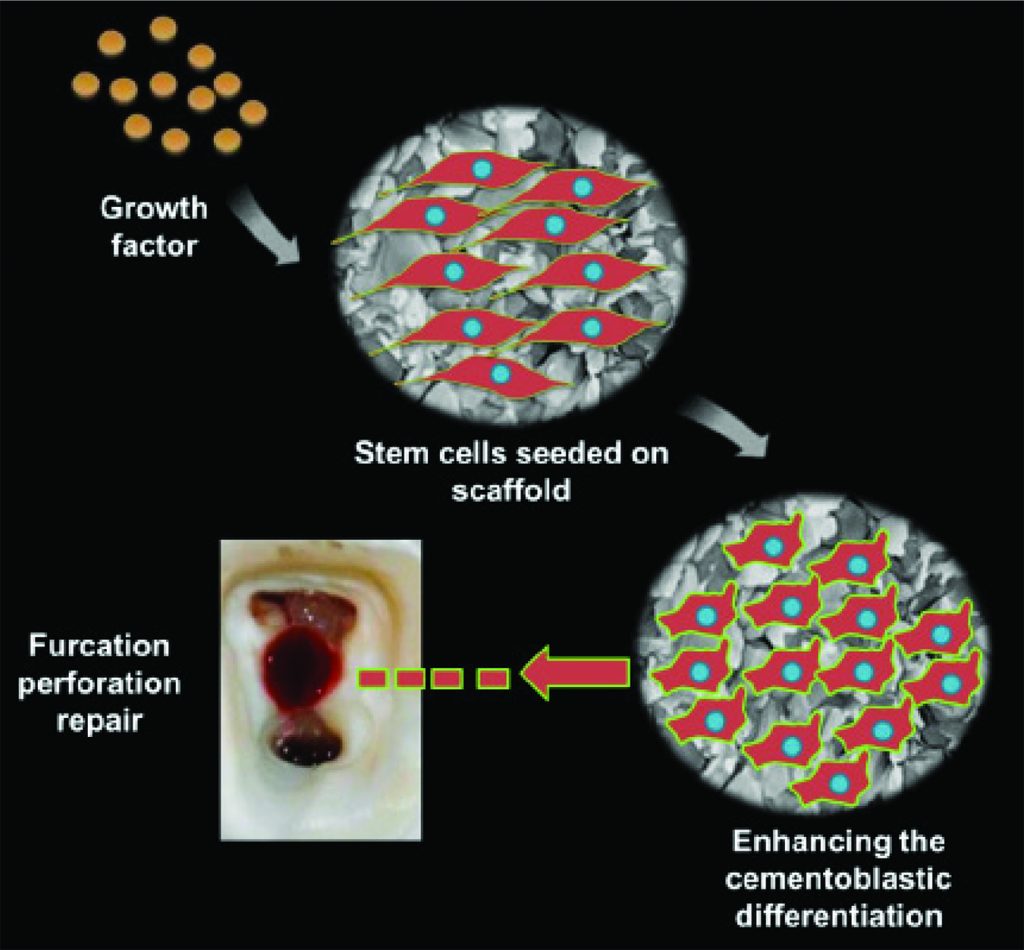
Figure 4: This illustration depicts a furcation perforation repair using stem cells, scaffold, and growth factor. This method has the potential to open new avenues in furcation repair treatment in the foreseeable near future. This image relates to the text under “future perspectives for the perforation repair”
Non-surgical approach
When a perforation repair is indicated, it is recommended to first attempt an intra-coronal approach (non-surgical) to preserve the periodontium, thus increasing the chances of success (Regan, Witherspoon, Foyle, 2005). Generally, perforations coronal to the crestal bone fall into the category of a non-surgical approach. The use of a surgical microscope operated at high magnification and with ample illumination allows for better management of perforation repairs (Kratchman, 2004; Daoudi, Saunders, 2002).
A surgical approach may complicate the treatment and lead to loss of periodontal attachment, chronic inflammation, and furcal pocket formation (Arens, Torabinejad, 1996). Experience has shown that buccally located perforations are easier to repair than lingual or proximal lesions. Lingual located perforations, especially in the mandible, should be treated non-surgically or orthodontically. If they are not responding to treatment, the tooth should be extracted (Regan, et al., 2005). If a tooth can be extruded orthodontically to a point where the perforation reaches a supragingival level, repair of the defect will be greatly facilitated (Smidt, Lachish-Tandlich, Venezia, 2005). Whether clinically practical or not, one case of intentional reimplantation was reported after repair of the perforation was performed on the extracted tooth (Poi, et al., 1999).
In cases of large perforations, bleeding should be controlled first using sterile saline. Alternatively, calcium hydroxide, calcium sulfate, or collagen has been used (Clauder, Shin 2009). For bleeding control, nonspecific intravascular clotting agents should be avoided as they may lead to alveolar bone damage and delay in healing (Lemon, Steele, Jeansonne, 1993). In cases of perforations that are infected or perforation sites that need further enlargement and cleaning, burs or ultrasonic tips may be used. However, ultrasonic tips are preferable as they are gentler to the adjacent periodontium tissues (Pitt Ford, et al., 1995; Arens, Torabinejad, 1996; Clauder, Shin, 2009). For cleaning of infected perforations, 2.5% sodium hypochlorite has been used (Arens, Torabinejad, 1996); however, sterile saline is indicated in large perforations (Clauder, Shin, 2009). To avoid blockage of the canals with repair material, gutta-percha points, paper points, cotton pellets, or an easily removable material (such as Cavit™, 3M) should be placed over the canal orifices (Clauder, Shin, 2009).
A resin-bonded material such as Geristore® (DenMat) is recommended to restore subgingival defects (Clauder, Shin, 2009), which also serves as an adjunct to GTR (Abitbol, et al., 1996; Behnia, Strassler, Campbell, 2000). It is less sensitive to moisture than conventional glass ionomer cement, while a drier environment improved the results (Cho, Kopel, White, 1995). Adhesive materials can be used in supracrestal perforations, whereas MTA is preferable in subcrestal perforations (Clauder, Shin, 2009). If a perforation defect involves bone destruction (intraosseus defect), a barrier is needed to facilitate controlled placement of the repair material. This is not necessaary if the defect does not include an intraosseus defect (Clauder, Shin, 2009). If MTA is used, a moist cotton pellet should cover the material to allow setting of the material. After perforation repair, the final restoration can be placed either after 1 day or 1 week. Once repair has been achieved, the root canal(s) can be cleaned, shaped, and filled (Pitt Ford, et al., 1995; Arens, Torabinejad, 1996).
If a perforation is present in the middle third of the root, the canal(s) should be prepared first before closing the defect to avoid blocking the canal. With the aid of an operating microscope, obturation of the canal apical to the defect should be done first, followed by filling the remainder of the canal and the perforation site with MTA (Clauder, Shin, 2009). Alternatively, the root space beyond the perforation can be maintained by means of a file or gutta-percha cone. In case a file is used, it should be loosened after finishing the repair procedure to allow easy removal before the MTA is fully set (Clauder, Shin, 2009). The other option is to use a gutta-percha point and soften it with heat to the dentinal wall opposing the perforation. MTA is then placed at the defect site (Clauder, Shin, 2009). Perforations at the apical one-third are quite challenging and difficult to manage. Successful treatment cannot always be achieved for all cases necessitating apical surgery or extraction of the tooth to remedy the problem (Clauder, Shin, 2009).
Surgical approach
Surgical intervention (external approach) is indicated in areas that are not accessible by non-surgical means alone, cases that have not responded to non-surgical treatment, or in repairing a perforating resorption (Regan, et al., 2005). The surgical approach is performed by reflecting a flap at the perforation site followed by cleaning and preparing the perforated area and finally packing the repair material (Alhadainy, 1994).
During the surgical repair procedures, cortical bone damage is involved, which may result in reduced success of the corrective surgical procedure. Thus, a GTR technique has been recommended for successful treatment outcomes by using either non-resorbable or resorbable membranes as a barrier (Duggins, et al., 1994; Barkhordar, Javid 2000; Rankow, Krasner, 1996; Dean, et al., 1997; Leder, et al., 1997). This barrier guides selected cells to populate at the perforation defect — i.e., placing the barrier between the gingival tissue and the perforation defect — will facilitate the repopulation of the defect by periodontal ligament cells and other osteogenic cells and prevents the colonization by gingival cells (Linde, et al., 1993; Sandberg, Dahlin, Linde, 1993). A resorbable membrane is generally preferable, as it does not need a second surgical procedure to remove it. However, in some cases, titanium-tented membrane or a supporting graft material is needed to prevent collapsing the membrane into the defect (Abitbol, et al., 1996).
 Figure 5: MTA-Angelus
Figure 5: MTA-Angelus
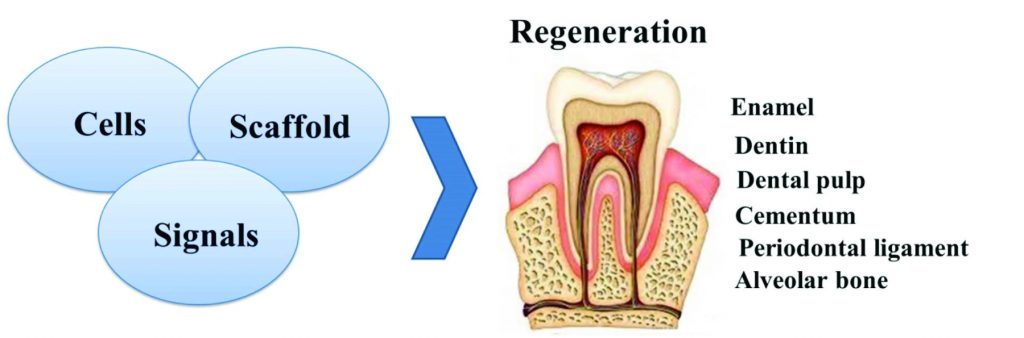
Figure 6: The three key elements of dental tissue engineering are stem cells, scaffolds, and signals
Cementum regeneration and role in the periodontium reconstruction
Cementum formation is very essential in the furcation perforation repair process (Pitt Ford, et al., 1995; Clauder, Shin, 2009; Samiee, et al., 2010; Zairi, et al., 2012). Pitt Ford and colleagues (1995) evaluated the histologic response to experimentally induced furcation perforations in dog mandibular premolars repaired by either MTA or amalgam and found that most of the MTA samples showed no inflammation and cementum deposition, whereas with the use of amalgam, moderate to severe inflammation with no cementum deposition was present.
Healing after intentional perforations in dogs’ teeth was evaluated after repair with either MTA or Sealapex™ (Kerr) (Holland, et al., 2001). Most samples sealed with MTA showed new cementum deposition and an absence of inflammation. In 2010, Samiee and colleagues reported that cementum-like hard tissue was formed using either MTA or CEM cement in the furcation perforation in dogs in the presence of a mild inflammatory response. The authors concluded that both materials showed a similar favorable biological response in furcation perforation repair.
Zairi, et al. (2012), compared the inflammatory reactions and tissue response of furcal perforations in dogs’ teeth to growth factors, TGFβ1, basic fibroblast growth factor (bFGF), osteogenic protein-1 (OP-1), and IGF-I, with MTA or IRM as controls. The authors reported that a clear stimulatory effect on cementum formation and inhibition of collagen capsule formation was exerted by the growth factors. However, MTA exhibited better results than the growth factors. Based on that, the authors suggested a further study comparing the effects of application of growth factor mixture with MTA and MTA alone on tissue healing and regeneration.
In a case report, Bains, et al. (2012), used tissue engineering principles for the furcation perforation repair of the pulpal floor of the right mandibular first molar of 39-year-old male patient using MTA and platelet-rich fibrin (PRF). The authors reported that this combination was able to repair the perforation defect and regenerate the lost perio-dontium in the furcation area effectively. A case report (Eghbal, Fazlyab, Asgary, 2014) was published describing the non-surgical endodontic management of an extensive perforation of the floor of the pulp chamber in a first mandibular molar of a 28-year-old Caucasian female using CEM cement. The authors reported that CEM was able to induce hard tissue formation, i.e., bone and cementum.
Cellular tissue engineering approach for cementum regeneration
A proposed therapeutic approach was reported by the removal of autologous cells from the patient’s periodontal ligament (PDL), culturing of the cells in vitro, which were then placed back onto the exposed root coated with chemo-attracting factors, subsequently covering the area with an artificial basement membrane (Terranova, 1990). However, it is unknown whether this method produced the desired effect. Lekic and colleagues (2005) reported that rat periodontal and bone marrow cells were able to differentiate into periodontal ligament fibroblasts, osteoblasts, and cementoblasts when transplanted into periodontal wounds in rats, thus contributing to periodontal regeneration.
Regeneration of cementum, PDL, and alveolar bone have been observed using auto-transplantation of bone marrow-derived mesenchymal stem cells (BM MSCs)(Kawaguchi, et al., 2004) or periodontal ligament cell sheet (Akizuki, et al., 2005) into periodontal osseous defects in dogs. However, the principle disadvantage of cell sheets is their delicate structure and difficult handling during surgery (Li, Jin, 2015). Furthermore, the harvest of bone marrow (BM) is a highly invasive and a painful procedure for the donor. Moreover, it has been reported that the number, proliferation, and differentiation potential of BM MSCs decline with increasing age (Kern, et al., 2006).
It has been reported that cementoblast-biodegradable poly(lactic-co-glycolic acid) (PLGA) polymer sponge-treated defects showed complete bone bridging and PDL formation, whereas minimal evidence of osteogenesis was exhibited by follicle cell-treated defects along the root surface of athymic rats (Zhao, et al., 2004). Perio-dontal ligament stem cells (PDLSCs) have the ability to differentiate into cementoblast and osteoblast (Isaka, et al., 2001; Seo, et al., 2004) and have shown potential therapeutic applications in periodontium regeneration. However, the very low number of these cells residing in the PDL is indicative of the difficulty acquiring a sufficient number for regenerative treatment remains and is an issue that remains unresolved (Maeda et al, 2011). Primary cultures of PDLSCs yielded small cell numbers, therefore before application, PDLSCs must proliferate at least 12 population doublings (Zhu, Liang, 2015). Additionally, it has been found that the proliferation and migration ability and differentiation potential of PDLSCs decreased with increasing age (Zhu, Liang, 2015).
Apical tooth germ cells conditioned medium were able to provide the cementogenic microenvironment and induced the cementoblastic differentiation of PDLSCs (Yang, et al., 2009). Hertwig’s epithelial root sheath (HERS) cells, or their secreted products, were able to induce PDL cells differentiation along the cementoblastic lineage in vitro (Zeichner-David, et al., 2003). Several in vivo studies have also shown the potential capability of PDLSCs to form cementum and PDL-like tissues (Yang, et al., 2009; Liu, et al., 2008; Feng, et al., 2010; Park, Jeon, Choung, 2011).
Regenerative therapy
Tissue engineering is an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ (Langer, Vacanti, 1993). Tissue engineering aims to stimulate the body either to regenerate tissue on its own or to grow tissue outside the body, which can then be implanted as natural tissue (Nadig, 2009).
Triad components
Regenerative endodontics can be defined as biologically based procedures designed to replace damaged structures, including dentin and root structures, as well as cells of the pulp-dentin complex (Murray, Garcia-Godoy, Hargreaves, 2007). This approach consists of the following interactive triad: 1) an appropriate cell source; 2) a supportive matrix (scaffold); and 3) inductive biological factors or signals (Figure 4). To create regenerative therapies, these disciplines are often combined rather than used individually (Murray, Garcia-Godoy, Hargreaves, 2007).
Future perspectives for the perforation repair
Reconstruction of the lost attachment via regeneration of the periodontium components, such as cementum, PDL, and bone, is essential in the repair of perforated areas. Replacement of the lost cementum (cementogenesis) is very critical and enhances the reattachment of the fibers of the periodontal ligament. Several studies have been published that demonstrate the ability of different materials to repair furcation perforations, albeit with variable success rates.
However, during recent years, there has been a paradigm shift from conventional to regenerative endodontic therapy, and repair of the periodontium is not an exception. To date, to the best of our knowledge, no studies have been published in the literature reporting on the effect of the triad application (stem cells, scaffold, and growth factor) for furcal perforation repair and the response of surrounding tissues (cementum, PDL, and alveolar bone). We propose a stem cell-based tissue engineering approach for furcation perforation repair through enhancing of stem cell differentiation along the cementoblastic lineage in association with scaffold and growth factor. The suggested biomimetic approach is illustrated in Figure 6. This will have the potential to open a new era and strategy in endodontic and periodontal tissue-engineering therapies.
Conclusions
Perforation of the pulp chamber floor of multi-rooted teeth constitutes a perplexing and frustrating problem. It is a major cause of endodontic treatment failure. A furcation perforation has to be regarded as an endodontic and periodontal problem. The inflammatory response in the periodontium, leading to irreversible loss of periodontal attachment in the area, can result in loss of the tooth if the perforation is not successfully repaired. To re-establish the periodontal tissue in the perforation site, surgical and non-surgical techniques have been utilized.
For furcation perforation repair, several materials have been used with varying results. However, the stem cell-based tissue engineering approach is very promising and is suitable for furcation perforation repair. This approach has the potential to revolutionize the practice of regenerative endodontics in the future, and may therefore, save many teeth that would otherwise have to be extracted due to a poor to hopeless prognosis. Moreover, it will help and assist in designing regenerative therapies based on sound biological principles, which can be applied in both endodontic and periodontal specialties.
Acknowledgments
This study was financially supported by the Universiti Sains Malaysia Research University Grant 1001/ PPSP/813058, PRGS (1001/PPSG/8146005) and short-term grants (304/PPSG/ 61312012 and 304/ PPSG/61312018) from the School of Dental Sciences, Universiti Sains Malaysia.
References
- Abitbol T, Santi E, Scherer W, Palat M. Using a resin-ionomer in guided tissue regenerative procedures: technique and application–case reports. Periodontal Clin Investig. 1996;18(1):17-21.
- Akizuki T, Oda S, Komaki M, et al. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res. 2005; 40(3):245-251.
- Al-Daafas A, Al-Nazhan S. Histological evaluation of contaminated furcal perforation in dogs’ teeth repaired by MTA with or without internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(3):92-99.
- Alhadainy HA. Root perforations. A review of literature. Oral Surg Oral Med Oral Pathol. 1994;78(3):368-374.
- American Association of Endodontists. Glossary of Endodontic Terms. Chicago, Illinois; 2003
- Amini Ghazvini S, Abdo Tabrizi M, Kobarfard F, Akbarzadeh Baghban AR, Asgary S. Ion release and pH of a new endodontic cement, MTA and Portland cement. Iranian Endod J. 2009;4(2):74-78.
- Apaydin ES, Shabahang S, Torabinejad M. Hard-tissue healing after application of fresh or set MTA as root end-filling material. J Endod. 2004;30(1):21-24.
- Arens DE, Torabinejad M. Repair of furcal perforations with mineral trioxide aggregate: two case reports. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(1):84-88.
- Asgary S, Akbari Kamrani F. Antibacterial effects of five root canal sealing materials. J Oral Sci. 2008;50(4):469-474.
- Asgary S, Eghbal MJ, Parirokh M, Torabzadeh H. Sealing ability of three commercial mineral trioxide aggregates and an experimental root-end filling material. Int Endod J. 2006;1(0):101-105.
- Asgary S, Eghbal MJ, Parirokh M. Sealing ability of a novel endodontic cement as a root-end filling material. J Biomed Mater Res A. 2008;87(3):706-709.
- Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31(2):101-103.
- Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histological response to different pulp capping materials and a novel experimental cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(4):609-614.
- Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009;35(3):147-152.
- Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate’s composition with Portland cements and a new endodontic cement. J Endod. 2009;35(2):243-250.
- Asgary S, Ehsani S. Permanent molar pulpotomy with a new endodontic cement: a case series. J Conserv Dent. 2009;12(1):31-36.
- Asgary S, Moosavi SH, Yadegari Z, Shahriari S. Cytotoxic effect of MTA and CEM cement in human gingival fibroblast cells. scanning electronic microscope evaluation. NY State Dent J. 78(2):51-54.
- Asgary S, Parirokh M, Eghbal MJ, Brink F. A comparative study of white mineral trioxide aggregate and white Portland cements using X-ray microanalysis. Aust Endod J. 2004;30(3):89-92.
- Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34(8):990-993.
- Bains R, Bains V, Loomba K, Verma K, Nasir A. Management of pulpal floor perforation and grade II furcation involvement using mineral trioxide aggregate and platelet rich fibrin: a clinical report. Contemp Clin Dent. 2012;3(suppl 2):223-227.
- Balla R, LoMonaco CJ, Skribner J, Lin LM. Histological study of furcation perforations treated with tricalcium phosphate, hydroxylapatite, amalgam, and life. J Endod. 1991;17(6):234-238.
- Bargholz C. Perforation repair with mineral trioxide aggregate: a modified matrix concept. Int Endod J. 2005;38(1):59-69.
- Barkhordar RA, Javid B. Treatment of endodontic perforations by guided tissue regeneration. Gen Dent. 2000;48(4):422-426.
- Behnia A, Strassler HE, Campbell R. Repairing iatrogenic root perforations. J Am Dent Assoc. 2000;131(2):196-201.
- Bryan EB, Woollard G, Mitchell WC. Nonsurgical repair of furcal perforations: a literature review. Gen Dent. 1999;47(3):274-278.
- Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31(9):665-668.
- Cho E, Kopel H, White SN. Moisture susceptibility of resin-modified glass-ionomer materials. Quintessence Int. 1995;26(5):351-358.
- Clauder T, Shin SU. Repair of perforations with MTA: clinical applications and mechanisms of action. Endod Topics. 2009;15:32-55.
- Coomaraswamy KS, Lumley PJ, Hofmann MP. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement based (MTA-like) system. J Endod. 2007;33(3):295-298.
- Daoudi MF, Saunders WP. In vitro evaluation of furcal perforation repair using mineral trioxide aggregate or resin modified glass lonomer cement with and without the use of the operating microscope. J Endod. 2002;28(7):512-515.
- De-Deus G, Petruccelli V, Gurgel-Filho E, Coutinho-Filho T. MTA versus Portland cement as repair material for furcal perforations: a laboratory study using a polymicrobial leakage model. Int Endod J. 2006;39(4):293-298.
- Dean JW, Lenox RA, Lucas FL, Culley WL, Himel VT. Evaluation of a combined surgical repair and guided tissue regeneration technique to treat recent root canal perforations. J Endod. 1997;23(8):525-532.
- Duggins LD, Clay JR, Himel VT, Dean JW. A combined endodontic retrofill and periodontal guided tissue regeneration technique for the repair of molar endodontic furcation perforations: report of a case. Quintessence Int. 1994;25(2):109-114.
- Eghbal MJ, Fazlyab M, Asgary S. Repair of an extensive furcation perforation with CEM cement: a case study. Iran Endod J. 2014;9(1):79-82.
- Feng F, Akiyama K, Liu Y, et al. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16(1):20-28.
- Ferris DM, Baumgartner JC. Perforation repair comparing two types of mineral trioxide aggregate. J Endod. 2004; 30(6):422-424.
- Haghgoo R, Niyakan M, Nazari Moghaddam K, Asgary S, Mostafaloo N. An in vitro comparison of furcal perforation repaired with Pro-root MTA and new endodontic cement in primary molar teeth – a microleakage study. J Dent (Shiraz). 2014;15(1):28-32.
- Hamad HA, Tordik PA, McClanahan SB. Furcation perforation repair comparing gray and white MTA: a dye extraction study. J Endod. 2006;32(4):337-340.
- Hashem AA, Hassanien EE. ProRoot MTA, MTA-Angelus and IRM used to repair large furcation perforations: sealability study. J Endod. 2008;34(1):59-61.
- Holland R, Filho JA, de Souza V, Nery MJ, Bernabe PF, Junior ED. Mineral trioxide aggregate repair of lateral root perforations. J Endod. 2001;27(4):281-284.
- Imura N, Otani SM, Hata G, Toda T, Zuolo ML. Sealing ability of composite resin placed over calcium hydroxide and calcium sulphate plugs in the repair of furcation perforations in mandibular molars: a study in vitro. Int Endod J. 1998;31(2):79-84.
- Isaka J, Ohazama A, Kobayashi M, et al. Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol. 2001;72(3):314-323.
- Jantarat J, Dashper SG, Messer HH. Effect of matrix placement on furcation perforation repair. J Endod. 1999; 25(3):192-196.
- Kawaguchi H, Hirachi A, Hasegawa N, et al. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75(9):1281-1287.
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294-1301
- Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32(6):569-572.
- Kratchman SI. Perforation repair and one-step apexification procedures. Dent Clin North Am. 2004;48(1):291-307.
- Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260(5110):920-926.
- Leder AJ, Simon BI, Deasy M, Fenesy KE, Dunn S. Histological, clinical, and digital subtraction radiographic evaluation of repair of periodontal defects resulting from mechanical perforation of the chamber floor using ePTFE membranes. Periodontal Clin Invest. 1997;19:9-15.
- Lekic PC, Nayak BN, Al-Sanea R, Tenenbaum H, Ganss B, McCulloch C. Cell transplantation in wounded mixed connective tissues. Anat Rec A Discov Mol Cell Evol Biol. 2005;287(2):1256-1263.
- Lemon RR. Nonsurgical repair of furcation defects. Internal matrix concept. Dent Clin North Am. 1992;36(2):439-457.
- Lemon RR, Steele PJ, Jeansonne BG. Ferric sulfate hemostasis: effect on osseous wound healing. Left in situ for maximum exposure. J Endod. 1993;19(4):170-173.
- Li B, Jin Y. Periodontal tissue engineering: current approaches and future therapies. Tissue Eng Part B Rev. 2010;16(2):219-255.
- Linde A, Alberius P, Dahlin C, Bjurstam K, Sundin Y. Osteopromotion: a soft-tissue exclusion principle using a membrane for bone healing and bone neogenesis. J Periodontol. 1993;64(suppl 11):1116-1128.
- Liu Y, Zheng Y, Ding G, Fang D, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26(4):1065-1073.
- Maeda H, Tomokiyo A, Fujii S, Wada N, Akamine A. Promise of periodontal ligament stem cells in regeneration of periodontium. Stem Cell Res Ther. 2011;2(4):33.
- Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30(2):80-83.
- Mehrvarzfar P, Dahi-Taleghani A, Saghiri MA, et al. The comparison of MTA, Geristore® and Amalgam with or without Bioglass as a matrix in sealing the furcal perforations (in vitro study). Saudi Dent J. 2010;22(3):119-124.
- Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33(4):377-390.
- Nadig RR. Stem cell therapy – Hype or hope? A review J Conserv Dent 2009;12(14):131-138.
- Nakata T, Bae K, Baumgartner J (1998) Perforation repair comparing mineral trioxide aggregate and amalgam using an anaerobic bacterial leakage model. J Endod.24(0):184-186.
- Noetzel J, Ozer K, Reisshauer BH, et al. Tissue responses to an experimental calcium phosphate cement and mineral trioxide aggregate as materials for furcation perforation repair: a histological study in dogs. Clin Oral Investig. 2006;10(1):77-83.
- Oliveira MG, Xavier CB, Demarco FF, Pinheiro AL, Costa AT, Pozza DH. Comparative chemical study of MTA and Portland cements. Braz Dent J. 2007;18(1):3-7.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
- Park, JY, Jeon, SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20(2):271-85.
- Pitt Ford TR, Torabinejad M, McKendry DJ, Hong CU, Kariyawasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(0):756-763.
- Poi WR, Sonoda CK, Salineiro SL, Martin SC. Treatment of root perforation by intentional reimplantation: a case report. Endod Dent Traumatol. 1999;15(3):132-134.
- Rafter M, Baker M, Alves M, Daniel J, Remeikis N. Evaluation of healing with use of an internal matrix to repair furcation perforations. Int Endod J. 2002;35(9):775-783.
- Rankow HJ, Krasner PR. Endodontic applications of guided tissue regeneration in endodontic surgery. J Endod. 1996;22(1):34-43.
- Regan JD, Witherspoon DE, Foyle DM. Surgical repair of root and tooth perforations. Endod Topics. 2005;11:152-178.
- Roda RS. Root perforation repair: surgical and nonsurgical management. Pract Proced Aesthet Dent. 2001; 13(6):467-472.
- Salman MA, Quinn F, Dermody J, Hussey D, Colaffey N. Histological evaluation of repair using a bioresorbable membrane beneath a resin-modified glass ionomer after mechanical furcation perforation in dogs’ teeth. J Endod. 1999;25(3):181-186.
- Samiee M, Eghbal MJ, Parirokh M, Abbas FM, Asgary S. Repair of furcal perforation using a new endodontic cement. Clin Oral Investig. 2010;14(6):653-658.
- Sandberg E, Dahlin C, Linde A. Bone regeneration by the osteopromotion technique using bioabsorbable membranes: an experimental study in rats. J Oral Maxillofac Surg 1993;51(10):1106-1114.
- Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31(2):97-100.
- Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149-155.
- Sinai.1977; Endodontic perforations: their prognosis and treatment. J Am Dent Assoc. 1977;95(1):90-95.
- Sinai IH, Romea DJ, Glassman G, Morse DR, Fantasia J, Furst ML. An evaluation of tricalcium phosphate as a treatment for endodontic perforations. J Endod. 1989;15(9):399-403.
- Sluyk SR, Moon PC, Hartwell GR. Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod. 1998;24(11):768-771.
- Smidt A, Lachish-Tandlich M, Venezia E. Orthodontic extrusion of an extensively broken down anterior tooth: a clinical report. Quintessence Int. 2005;36(2):89-95.
- Taneja S, Kumari M. Effect of internal matrices of hydroxyapatite and calcium sulfate on the sealing ability of mineral trioxide aggregate and light cured glass ionomer cement. J Conserv Dent. 2011;14(1):6-9.
- Terranova VP. Periodontal and bone regeneration factor, materials and methods. 1990; International patent WO 90/ 100017
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999; 25(3):197-205.
- Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: effects of blood contamination. J Endod. 1994;20(4):159-163.
- Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21(0):603-608.
- Torabinejad M, Hong CU, McDonald F, Pitt Ford TR (1995) Physical and chemical properties of a new root-end filling material. J Endod.21(0):349-353.
- Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19(12):591-595.
- Tronstad L, Wennberg A. In vitro assessment of the toxicity of filling materials. Int Endod J. 1980;13(3):131-138.
- Tsesis I, Fuss Z. Diagnosis and treatment of accidental root perforations. Endod Topics. 2006;13:95-107.
- Yang ZH, Zhang XJ, Dang NN, et al. Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J Periodontal Res. 2009; 44(2):199-210.
- Yildirim T, Gencoglu N, Firat I, Perk C, Guzel O. Histologic study of furcation perforations treated with MTA or Super-EBA in dog’s teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(1):120-124.
- Zairi A, Lambrianidis T, Pantelidou O, Papadimitriou S, Tziafas D. Periradicular tissue responses to biologically active molecules or MTA when applied in furcal perforation of dogs’ teeth. Int J Dent 2012;1-9.
- Zeichner-David M, Oishi K, Su Z, et al. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev Dyn. 2003;228(4):651-663.
- Zhao M, Jin Q, Berry JE, Nociti FH Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75(1):154-161.
- Zhu W, Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cells Int. 2015;(2015):1-11
- Zhu YQ, Xia WW, Xia L. Histological evaluation of repair of furcation perforation in dogs using mineral trioxide aggregate. Shanghai Kou Qiang Yi Xue, 2003;12:47-50.
- Zou L, Liu J, Yin S, Li W, Xie J. In vitro evaluation of the sealing ability of MTA used for the repair of furcation perforations with and without the use of an internal matrix. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(0):661-65.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


