CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
This article aims to present a case of a tooth with pulp necrosis, periradicular lesion and severe inflammatory apical root resorption, where endodontic treatment was performed in a single session.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Recognize certain characteristics of the external inflammatory apical root resorption process.
- Realize differences in endodontic treatment with and without the use of an intracanal medication.
- Discuss the use of calcium hydroxide as an intracanal medication.
- Recognize the involvement of cementum and dentin in healing of external root resorption.
- Identify the steps that lead to the success of pulp necrosis and severe inflammatory apical root resorption in a single session after a 6-month follow-up.
Drs. Ricardo Machado, Emanuely da Silva Chrun, Luiz Fernando Tomazinho, and Lucas da Fonseca Roberti Garcia consider the possibility of endodontic treatment of a tooth with pulp necrosis and severe inflammatory external apical root resorption in a single session
Root resorption is characterized by an unregulated function between blastic and clastic cells, normally responsible for the maintenance and remodeling of the periodontal support tissues. This condition may lead to tooth loss from uncontrolled cell activity if adequate treatment is not given (Andreasen, 1985).
Particularly in regards to external inflammatory apical root resorption, several studies have shown a positive correlation between this disease, pulp necrosis, and the presence of periradicular lesions (Campos, et al., 2013; Vier-Pelisser, et al., 2013). Thus, performing proper endodontic treatment may interrupt the external inflammatory apical root resorption process by neutralizing microbiological content and inhibiting clastic action (Barratto-Filho, et al., 2009).
The number of sessions required to properly reduce the microbial population of a contaminated root canal system is still a controversial issue among researchers (Kvist, et al., 2004; Molander, et al., 2007; Paredes-Vieyra, Enriquez, 2012). In recent years, several clinical and meta-analysis studies have been performed to compare endodontic treatment with and without the use of an intracanal medication and have reported similar results between these two treatment modalities (Kvist, et al., 2004; Molander, et al., 2007; Soltanoff, 1978). However, to date, no conclusive scientific evidence has been found on required use of an intracanal medication in cases of pulp necrosis, periradicular lesion, and severe associated inflammatory apical root resorption.
Thus, the purpose of this article is to report the clinical case of a tooth with pulp necrosis, periradicular lesion, and severe inflammatory apical root resorption, where endodontic treatment was performed in a single session. The 6-month follow-up shows clear signs of repair.
Case report
A 24-year-old male patient was referred to the endodontic specialization course at Ingá University, UNINGÁ, Rio Branco, AC, Brazil, for analysis of tooth No. 36. The patient related episodes of pain and swelling in this region a few months prior. Clinical examination revealed an extensive carious lesion and provisional sealing with temporary restorative material. Radiographic analysis showed communication of the temporary restorative material with the pulp chamber, periradicular lesions in both roots, and severe inflammatory apical root resorption in the distal root (Figure 1A).
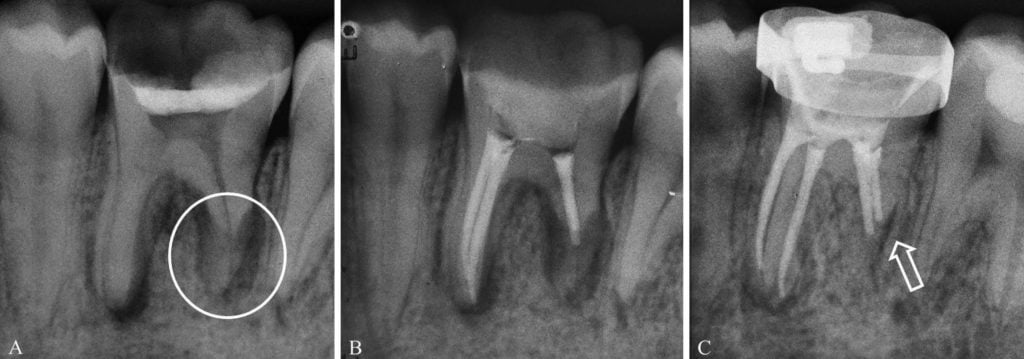
Figures 1A-1C: A. Initial periapical radiograph of tooth No. 36, showing periapical lesions in both roots, and severe apical root resorption in the distal root (circle). B. Tooth No. 36 after filling of root canal system. C. Radiograph after 6-month follow-up, with clear evidence of tissue repair and containment of the resorptive process (arrow)
After conducting clinical and radiographic analysis, it was decided that endodontic treatment should be performed. Initially, the tooth was anesthetized with 4% articaine and adrenaline 1:100.000 (DFL Indústria e Comércio), followed by the placement of a rubber dam. Then the temporary restorative material and the carious lesion were removed with spherical 1016 and Endo™ Z burs (KG Sorensen) coupled with a high-speed device (Extra Torque 605C, KaVo). Four canal orifices were identified with endodontic probes (MB, ML, DB, and DL) and prepared with number two Gates-Glidden burs (Dentsply Sirona). Then each canal was irrigated with 2.5 ml of 2.5% sodium hypochlorite (Fórmula & Ação).
Afterwards, the working lengths were established at -0.5 (mesial canals) and -1.0 mm (distal canals) from the point indicated by the electronic apex locator (Mini Apex Locator, SybronEndo) as “0.0.”
A manual glide path was created in the mesial root canals, with size 15 and 20 K-type files (Dentsply/Maillefer), followed by preparation using the two-system (VDW) full-sequence technique. The size of the distal canals required manual preparation to be performed up to size 60 K-type file (Dentsply/Maillefer), following the principles of the crown-down technique. The patency of the root canals was maintained by using a size 20 K-type file (Dentsply/Maillefer) up to the main foramen. The canals were irrigated at each change of file, with 2.5 ml of 2.5% sodium hypochlorite, and a final irrigation was performed with 2.5 ml of 17% EDTA (Fórmula & Ação) for 3 minutes to remove the smear layer.
The root canals were dried with absorbent paper cones (Dentsply/Maillefer) and filled with gutta-percha cones (Dentsply/Maillefer) and Sealer 26 (Dentsply/Maillefer) (Figure 1B), using the lateral compaction technique.
Six months after the treatment, the patient returned for a follow-up and related no pain or any relevant symptomatology. Radiographic examination showed clear evidence of tissue repair and containment of the resorptive process (Figure 1C).
Discussion
Since no evidence of dental trauma, occlusal disharmony, or relevant associated systemic disease was observed in this case report, it was concluded that the severe inflammatory resorptive process evolved from carious lesion to pulp necrosis and periradicular disease. Complete necrosis of pulp tissue leads to colonization and proliferation of microorganisms within the root canal system, inducing periradicular inflammation, which promotes clastic cell activity, and, in turn, triggers an osseous and radicular resorptive process (Patel, et al., 2009).
Some studies have advocated the use of calcium hydroxide as an intracanal medication in cases of open apices caused by incomplete apexogenesis, over-instrumentation, and/or apical resorptions (Mente, et al., 2009; Mente, et al., 2013). In addition to its antimicrobial activity, this substance acts as a physical-chemical barrier, preventing the proliferation of residual microorganisms, reinfection of the root canal by microorganisms originating from the oral cavity, and invagination of the granulation tissue of the area reabsorbed by the walls of the root canal. Furthermore, calcium hydroxide is capable of promoting necrosis of the resorptive cells present in Howship’s lacunae, thus neutralizing clastic cell acids, preventing the mineral dissolution of the root, and rendering the region unsuitable for acid hydrolases (Mohammadi, Dummer, 2011; Saad, 1989).
Healing of external root resorption, involving cementum and dentin caused by apical periodontitis also requires the recruitment of progenitor cells. Dentin-producing odontoblasts can be differentiated only from dental pulp stem cells (Gronthos, et al., 2000), and stem cells, from apical papilla (Sonoyama, et al., 2008). In mature teeth with apical periodontitis, the dental pulp is completely destroyed, and the apical papilla no longer exists. In addition, stem cells/progenitor cells in the periodontal ligament and alveolar bone marrow are not capable of differentiating into odontoblasts (Huang, Gronthos, Shi, 2009; Seo, et al., 2004). Therefore, the resorbed root dentin caused by the inflammatory process cannot be regenerated by odontoblasts and dentin formation (Ricucci. et al., 2014).
Resorbed root dentin is repaired by cementum and not by dentin (Lindskog, Blomlof, Hammarstrom, 1987). The mechanisms of repair by cementum formation, including the origin of cementoblasts and the molecules related to their recruitment and differentiation, remain unclear (Grzesik, Narayanan, 2002). Cementoblast progenitors have their origin in the periodontal ligament (usually in a paravascular location) or the endosteum (Liu, et al., 1997; McCulloch, 1993). In periradicular tissue healing, periodontal ligament cells adjacent to the affected root area may start to proliferate and populate the region in which the periodontal ligament and cementum were changed or lost by inflammation. It has been suggested that the cementum matrix and associated molecules can recruit cementum-forming stem/progenitor cells in the periodontal ligament (Grzesik, Narayana, 2002), and that the dentin matrix may also be able to signal progenitor cells in the periodontal ligament (Diekwisch, 2001) to differentiate into cementoblasts. Initially, cementoblast progenitors have to be selected, possibly by specific integrins and signaling events (Grzesik, Narayanan, 2002; Wu, et al., 1996). Then the selected cells adhere to the root surface and are activated by growth factors previously sequestered in the cementum and dentin matrix and released as a consequence of root resorption. These factors include bone morphogenetic proteins, transforming growth factor beta, insulin-like growth factor one, and epidermal growth factor (Grzesik, Narayanan, 2002; MacNeil, Somerman, 1999). Newly formed cementum usually covers areas of the root where cementum and dentin were lost (Ricucci, et al., 2014).
It seems much more “plausible biologically” that the entire immunological complex is activated after performing an adequate cleaning and shaping process of the root canal system and not only or necessarily after using calcium hydroxide. This argument is based on the absence of statistically significant differences in the success rates of necrotic teeth with radiographically visualized periradicular lesions treated with or without the use of this substance as an intracanal medication (Molander, et al., 2007; Paredes- Vieyra, Enriquez, 2012; Penesis, et al., 2008).
With this in mind, it was decided that appropriate endodontic treatment could be concluded in a single session, based on the certainty that correct cleaning and shaping could be performed, and that all the canals could be completely dried after this phase.
The success of this treatment was observed after the 6-month follow-up, at which time no pain, sinus tract, swelling, or discomfort was observed or related by the patient. Although it is thought that randomized clinical studies must be conducted to compare the results of endodontic treatment performed in a single or more sessions for teeth with pulp necrosis, periradicular lesion, and severe inflammatory apical root resorptions, the clinical case related in this article demonstrates the feasibility of performing endodontic treatments for these cases in a single visit.
References
- Andreasen JO. External root resorption: its implication in dental traumatology, paedodontics, periodontics, orthodontics and endodontics. Int Endod J. 1985;18(2):109-118.
- Baratto-Filho F, Leonardi DP, Zielak JC, Vanni JR, Sayao-Maia SM, Sousa-Neto MD. Influence of ProTaper finishing files and sodium hypochlorite on cleaning and shaping of mandibular central incisors – a histological analysis. J Appl Oral Sci. 2009;17(3):229-233.
- Campos MJ, Silva KS, Gravina MA, Fraga MR, Vitral RW. Apical root resorption: the dark side of the root. Am J Orthod Dentofacial Orthop. 2013;43(4):492-498.
- Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45(5-6):695-706.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625-13630.
- Grzesik WJ, Narayanan AS. Cementum and periodontal wound healing and regeneration. Crit Rev Oral Biol Med. 2002;13(6):474-484.
- Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792-806.
- Kvist T, Molander A, Dahlen G, Reit C. Microbiological evaluation of one- and two-visit endodontic treatment of teeth with apical periodontitis: a randomized, clinical trial. J Endod. 2004;30(8):572-576.
- Lindskog S, Blomlof L, Hammarstrom L. Cellular colonization of denuded root surfaces in vivo: cell morphology in dentin resorption and cementum repair. J Clin Periodontol. 1987;14(7):390-395.
- Liu HW, Yacobi R, Savion N, Narayanan AS, Pitaru S. A collagenous cementum-derived attachment protein is a marker for progenitors of the mineralized tissue-forming cell lineage of the periodontal ligament. J Bone Miner Res. 1997;12(10):1691-1699.
- MacNeil RL, Somerman MJ. Development and regeneration of the periodontium: parallels and contrasts. Periodontol 2000. 1999;19:8-20.
- McCulloch CA. Basic considerations in periodontal wound healing to achieve regeneration. Periodontol 2000. 1993;1(1):16-25.
- Mente J, Hage N, Pfefferle T, Koch MJ, Dreyhaupt J, Staehle HJ, Friedman S. Mineral trioxide aggregate apical plugs in teeth with open apical foramina: a retrospective analysis of treatment outcome. J Endod. 2009;35(10):1354-1358
- Mente J, Leo M, Panagidis D, et al. Treatment outcome of mineral trioxide aggregate in open apex teeth. J Endod. 2013;39(1):20-26.
- Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44(8):697-730.
- Molander A, Warfvinge J, Reit C, Kvist T. Clinical and radiographic evaluation of one-and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. J Endod. 2007;33(10):1145-1148.
- Paredes-Vieyra J, Enriquez FJ. Success rate of single- versus two-visit root canal treatment of teeth with apical periodontitis: a randomized controlled trial. J Endod. 2012;38(9):1164-1169.
- Patel S, Dawood A, Wilson R, Horner K, Mannocci F. The detection and management of root resorption lesions using intraoral radiography and cone beam computed tomography — an in vivo investigation. Int Endod J. 2009;42(9):831-838.
- Penesis VA, Fitzgerald PI, Fayad MI, Wenckus CS, BeGole EA, Johnson BR. Outcome of one-visit and two-visit endodontic treatment of necrotic teeth with apical periodontitis: a randomized controlled trial with one-year evaluation. J Endod. 2008;34(3):251-257.
- Ricucci D, Siqueira JF Jr, Loghin S, Lin LM. Repair of extensive apical root resorption associated with apical periodontitis: radiographic and histologic observations after 25 years. J Endod. 2014;40(8):1268-1274.
- Saad AY. Calcium hydroxide in the treatment of external root resorption. J Am Dent Assoc. 1989;118(5):579-581.
- Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149-155.
- Soltanoff W. A comparative study of the single-visit and the multiple-visit edodontic procedure. J Endod. 1978;4(9):278-281.
- Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod.2008;34(2):166-171.
- Vier-Pelisser FV, de Figueiredo JA, Reis Só MV, Estivallet L, Eickhoff SJ. Apical resorption in teeth with periapical lesions: correlation between radiographic diagnosis and SEM examination. Aust Endod J. 2013;39(1):2-7.
- Wu D, Ikezawa K, Parker T, Saito M, Narayanan AS. Characterization of a collagenous cementum-derived attachment protein. J Bone Miner Res. 1996;11(5):686-692.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


