Dr. Edward S. Lee discusses a tissue-engineering material with a variety of applications
Introduction
Platelet-rich fibrin (PRF) is a bioactive material made from the centrifugation of a patient’s whole blood. It is a second-generation platelet concentrate first described by Choukroun1 and is used to accelerate soft and hard tissue healing. This simplified and cost-effective chairside procedure results in a resorbable fibrin matrix enriched with platelets and leukocytes.
[userloggedin]
PRF provides a rich source of growth factors, including platelet-derived growth factors (PDGFs), transforming growth factors (TGFs), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF).2 The growth factors are slowly released during the course of the healing process.3 Because of the unique character of PRF, it is used as a tissue-engineering material with a wide range of dental applications.4,5 PRF is currently recommended as a scaffold material for regenerative endodontics.6
The following is a case study for its application in endodontics. Benefits and other applications are also discussed.
Case study
A 67-year-old female patient presented with mild discomfort and swelling around tooth No. 14. Her medical history was non-contributory. Teeth Nos. 12, 13, and 15 had been extracted more than 15 years previously and the area restored with a fixed bridge from teeth Nos. 11 to No. 14. The radiograph revealed lateral bone loss around the mesial buccal root with a 9 mm periodontal probing with probing WNL on other sites around the tooth. The patient was informed that she had a vertically fractured mesial buccal root (Figure 1). Treatment options were discussed, including extraction and replacement with implants or a removable partial denture. The patient decided to do a mesial buccal root amputation procedure and save the existing tooth and the bridge.
 Venous blood was drawn from the patient’s median cubital vein and collected in three 10 ml BD Vacutainer® (BD Worldwide) tubes without anticoagulants. The blood was immediately centrifuged at 1500 RPM for 10 minutes (MyRGF-I centrifuge, Boca Dental Supply, LLC). Three layers form in the tube:
Venous blood was drawn from the patient’s median cubital vein and collected in three 10 ml BD Vacutainer® (BD Worldwide) tubes without anticoagulants. The blood was immediately centrifuged at 1500 RPM for 10 minutes (MyRGF-I centrifuge, Boca Dental Supply, LLC). Three layers form in the tube:
- The lower fraction contains the RBCs.
- The middle fraction contains the PRF clot.
- The upper fraction contains the acellular platelet poor plasma (PPP) (Figure 2).
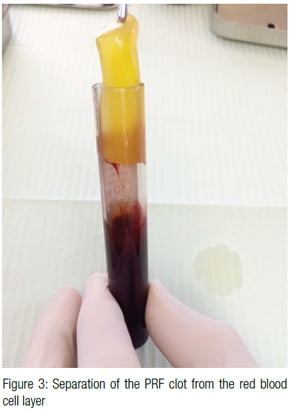 The middle portion containing the PRF clot was cut and removed from the RBC layer (Figure 3). The PRF clot was compressed to form a membrane using a membrane processing box (PRF MyRGF Box, Boca Dental Supply, LLC) (Figures 4, 5, 6). The liquid from the compressed membrane was collected and mixed with allograft bone (BoneBank Allograft, San Antonio, Texas). The collected liquid contains fibrin, which acts as a binder for the allograft bone that will be utilized and helps prevent graft migration from the intended site.
The middle portion containing the PRF clot was cut and removed from the RBC layer (Figure 3). The PRF clot was compressed to form a membrane using a membrane processing box (PRF MyRGF Box, Boca Dental Supply, LLC) (Figures 4, 5, 6). The liquid from the compressed membrane was collected and mixed with allograft bone (BoneBank Allograft, San Antonio, Texas). The collected liquid contains fibrin, which acts as a binder for the allograft bone that will be utilized and helps prevent graft migration from the intended site.
A full thickness trapezoidal flap with vertical releasing incisions mesial to No. 13 and distal to No. 14 was made. Upon reflection of the flap, a bone dehiscence over the mesial buccal root and a vertical root fracture were confirmed. A Lindemann bur was used to section the mesial buccal root at a supracrestal level, and a standard Class 1 root-end preparation was completed using ultrasonic instrumentation (ProUltra® Surgical Endo Tip, Dentsply Tulsa). The root was filled using a dentin bonding agent (Futurabond®, Voco Dental ), a flowable composite (Virtuoso® Flowable, Denmat), and polished with rubber points (Figure 7). The allograft bone mixture was placed into the bony defect followed by coverage with PRF membranes (Figures 8 and 9). Figure 10 shows the immediate postoperative radiograph. The sutures were removed after 1 week with minimal to no pain reported by the patient after the procedure.
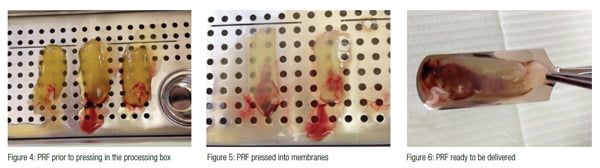
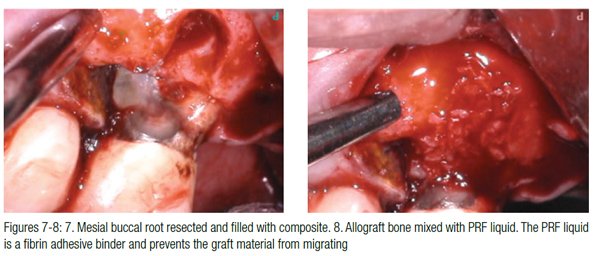
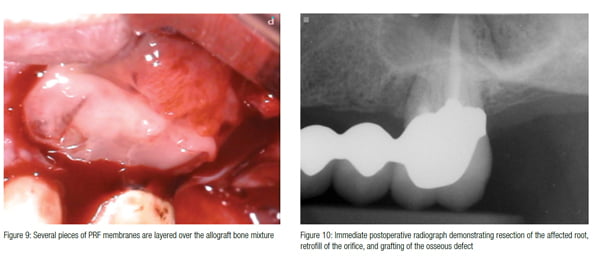
Figure 11 shows the 1-month healing. The area is healing with very little recession around the root amputation site. Figures 12 and 13 show the area after 7 months of healing. The bone has filled in, and the gingival architecture is excellent with no inflammation.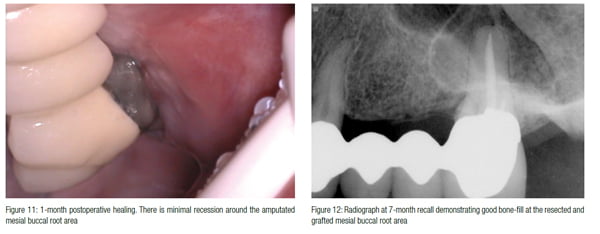
 Discussion
Discussion
Platelets play a crucial role not only in hemostasis but also in the wound-healing process.7 Growth factors contained within the α-granules of the platelets help regulate hard and soft tissue repair. These growth factors include platelet-derived growth factors (PDGFs), transforming growth factors (TGFs), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF). Leukocytes found in PRF also release growth factors, including TGFβ-1 and VEGF.3 The growth factors help promote cell migration, attachment, differentiation, and proliferation.2
Leukocytes cytokines, such as IL-1, IL-4, IL-6, and TNF-α, are also contained within PRF and help with immune and inflammatory regulation.8
PRF produces a significantly higher concentration of platelets and fibrin when compared to the initial input of whole blood volume.9 Growth factors within PRF are slowly and continuously released during healing.3
A key feature of the PRF process is the slow polymerization of fibrinogen into fibrin during centrifugation. A fine and flexible fibrin network forms resulting in an architecture that is characterized as a less rigid framework.10 PRF is a flexible, elastic, and resilient biomaterial able to support cytokines enmeshment and cellular migration.11
The high tear elastic modulus of PRF makes it suturable and functions much like a fibrin bandage.12 This helps promote wound closure and mucosal healing.
The compressed PRF liquid contains growth factors13 and proteins such as vitronectin and fibronectin.2 The PRF liquid can be mixed with allograft bone to help improve the handling characteristics of the graft and prevent graft migration during the healing process.
PRF is a more simplified technique compared with platelet rich plasma (PRP). PRP is a first-generation platelet-concentrate technique involving the use of anticoagulants, multiple centrifugation cycles, and surgical additives.14 The low-density fibrin network, short duration of growth factor release, potential allergic reaction to bovine thrombin additive, and cost make PRP much less attractive when compared to PRF.
Minsk and Polson15 suggested that root resection can be a valuable procedure when the tooth in question has a very high strategic value or when there are specific problems that cannot be solved by other therapeutic approaches. Teeth in proximity to anatomic landmarks, such as the maxillary sinus, can be treated safely by root resection therapy.
In this case study, bone grafting the root area after the mesial buccal root amputation helped maintain bone height and prevent ridge deficiency. The tooth has no mobility, and crestal bone levels are excellent in the months following the procedure. The 7-month follow-up shows good bone healing with full gingival architecture resulting in minimal food impaction and easier homecare.
Other applications of PRF in endo-dontics include regenerative endodontics,6 sinus membrane repair,16 perforation repair procedures,17 hard/soft tissue grafting of large defects, and socket preservation procedures.4,18,19, 20
Conclusion
Regenerative endodontics is a tissue-engineering process that requires the following three elements to succeed:
- Scaffold
- Growth factors
- Stem cells21
PRF provides a 100% bio-compatible, natural scaffold enriched with growth factor that can help with the proliferation and differentiation of human dental pulp cells.22
Further research and clinical trials are needed to understand the benefits and applications of PRF in endodontics.
Acknowledgment
The author would like to thank Dr. Calvin Tae Nam for his great help and inspiration.
[/userloggedin]
[userloggedout][/userloggedout]
- Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie.2001;42:55–62.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45–e50.
- Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63–69.
- Khiste SV, Tari RN. Platelet-Rich Fibrin as a Biofuel for Tissue Regeneration. ISRN Biomaterials. Vol. 2013, Article ID 627367, 6 pages, 2013. doi:10.5402/2013/627367.
- Borie E, García Oliví D, Orsi IA, Garlet K, Weber B, Beltrán V, Fuentes R. Platelet-rich fibrin application in dentistry: a literature review. Int J Clin Exp Med. 2015;8(5):7922–7929.
- American Association of Endodontists. AAE Clinical Considerations for Regenerative Procedure. Revised 4-12-15. https://www.aae.org/uploadedfiles/publications_and_research/research/currentregenerativeendodonticconsiderations.pdf. Accessed January 19, 2017.
- Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost.2006 May;4(5):932-939.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leukocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod.2006 Mar;101(3):e51-e5.
- Lucarelli E, Beretta R, Dozza B, Tazzari PL, O’Connel SM, Ricci F, Pierini M, Squarzoni S, Pagliaro PP, Oprita EI, Donati D. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater. 2010;20:13–23.
- Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Annals of the New York Academy of Sciences. 2001;936:11–30.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–e44.
- Khorshidi H, Raoofi S, Bagheri R, Banihashemi H. Comparison of the Mechanical Properties of Early Leukocyte- and Platelet-Rich Fibrin versus PRGF/Endoret Membranes. International Journal of Dentistry. Vol. 2016, Article ID 1849207, 7 pages, 2016. doi:10.1155/2016/1849207.
- Su CY, Kuo YP, Tseng YH, Su CH, Burnouf T. In vitro release of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Jul;108(1):56-61.
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol, Oral Radiol, Endod.1998;85(6): 638–646.
- Minsk SL, Polson AM. The role of root resection in the age of dental implants. Compend Contin Educ Dent. 2006 Jul; 27(7):384-388.
- Toffler M, Toscano N, Holtzclaw D, Corso MD, Dohan Ehrenfest DM. Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. J Implant Clin Adv Dent.2009;1:21–30.
- Bains R, Bains VK, Loomba K, Verma K, Nasir A. Management of pulpal floor perforation and grade II Furcation involvement using mineral trioxide aggregate and platelet rich fibrin: A clinical report. Contemp Clin Dent. 2012;Sep; 3(Suppl 2):S223–S227.
- Simonpieri A, Del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, Dohan Ehrenfest DM.. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol.2012;13(7):1231–1256.
- Singh S, Singh A, Singh S, Singh R. Application of PRF in surgical management of periapical lesions. Natl J Maxillofac Surg. 2013;4 (1): 94–99.
- Anantula K, Annareddy A. Platelet-rich fibrin (PRF) as an autologous biomaterial after an endodontic surgery: Case reports. J Dr NTR Univ Health Sci. 2016;5(1):49-54.
- Sharma S, Sikri V, Sharma NK, Sharma VM. Regeneration of tooth pulp and dentin : trends and advances. Annals of Neurosciences. 2010;17(1). https://annalsofneurosciences.org/journal/index.php/annal/article/viewArticle/ans.0972-7531.2010.170104/853. Accessed January 19, 2017.
- Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod.2010; 36(10):1628-1632.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Dr. Edward S. Lee has a private practice in San Francisco, California. He is an innovator in endodontics and is the inventor of the MTA Pellet Forming Block. To learn more about PRF, visit
Dr. Edward S. Lee has a private practice in San Francisco, California. He is an innovator in endodontics and is the inventor of the MTA Pellet Forming Block. To learn more about PRF, visit 
