Dr. Eoin Mullane examines the correct clinical use of antibacterial drugs in endodontics

 The discovery of safe, systemic antibiotics has been a major factor in the control of infectious diseases. As such, antibiotics have increased the life expectancy and quality of life for millions of people (AAE ENDODONTICS: Colleagues for Excellence, 2012).
The discovery of safe, systemic antibiotics has been a major factor in the control of infectious diseases. As such, antibiotics have increased the life expectancy and quality of life for millions of people (AAE ENDODONTICS: Colleagues for Excellence, 2012).
 But this life-altering benefit comes at a price — drug-resistant bacteria. The problem is not with the antibiotics themselves, but with how they are being used and, to a larger extent, prescribed (AAE ENDODONTICS: Colleagues for Excellence, 2012).
But this life-altering benefit comes at a price — drug-resistant bacteria. The problem is not with the antibiotics themselves, but with how they are being used and, to a larger extent, prescribed (AAE ENDODONTICS: Colleagues for Excellence, 2012).
A recent study, highlighted by the Irish media at the beginning of this year, found a 44-fold increase in the prevalence of MRSA strains and a sixfold increase in the number of strains resistant to multiple antibiotics (Kinnevey, et al., 2014).
 These bacterial strains were mostly associated with skin and soft tissue infections, and were found in patients with serious life-threatening illnesses.
These bacterial strains were mostly associated with skin and soft tissue infections, and were found in patients with serious life-threatening illnesses.
The dental profession also plays a part in the selection of drug-resistant bacterial strains, through inappropriate drug prescribing (Sweeney, et al., 2004). There is ever-increasing evidence from current endodontic literature of antibiotic resistance in endodontic infections (Jungermann, et al., 2011; Montagner, et al., 2014) — but there is a glimmer of hope.
By examining the following five myths, we can strive to achieve correct clinical use of antibacterial drugs and assist with clinical decisions regarding antibiotic therapy (AAE ENDODONTICS: Colleagues for Excellence, 2012).
The five myths
1. Antibiotics cure patients
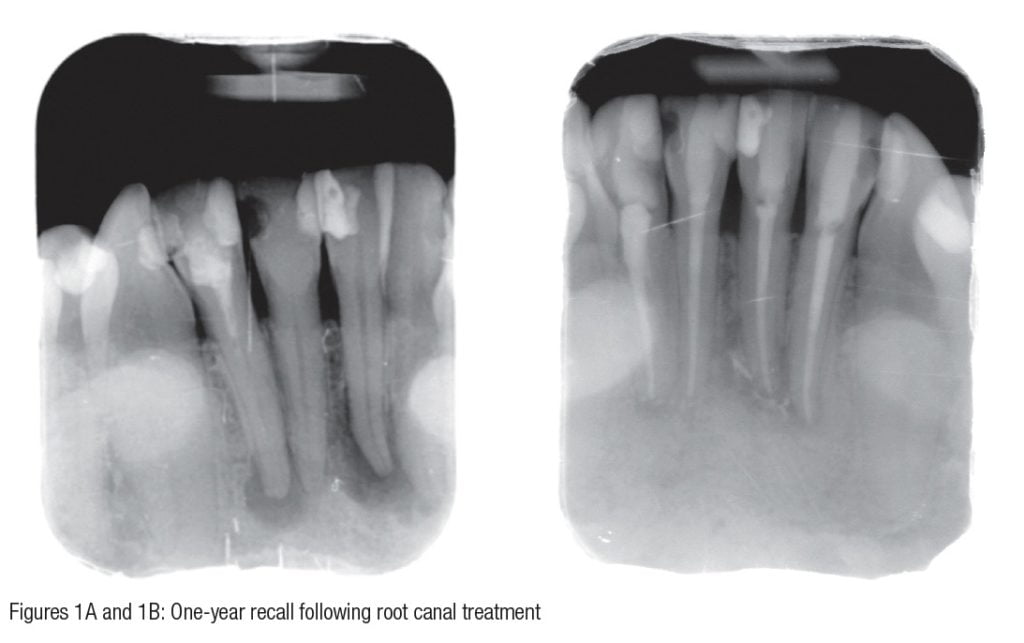
Antibiotics do not cure patients. Once a proper balance is re-established between the host defense (immune system and inflammatory system) and the bacterial agents, patients cure themselves (Figures 1A and 1B).
2. Antibiotics are a substitute for clinical intervention
It’s simple: Antibiotics are not a substitute for surgical intervention. Endodontics removes the source of infection; patients then heal themselves (Figures 2A and 2B).
3. The most important decision is which antibiotic to use
The most important decision is not which antibiotic to use, but whether or not we need to prescribe one at all. Most endodontic infections resolve once the source of the infection has been removed (AAE ENDODONTICS: Colleagues for Excellence, 2012).
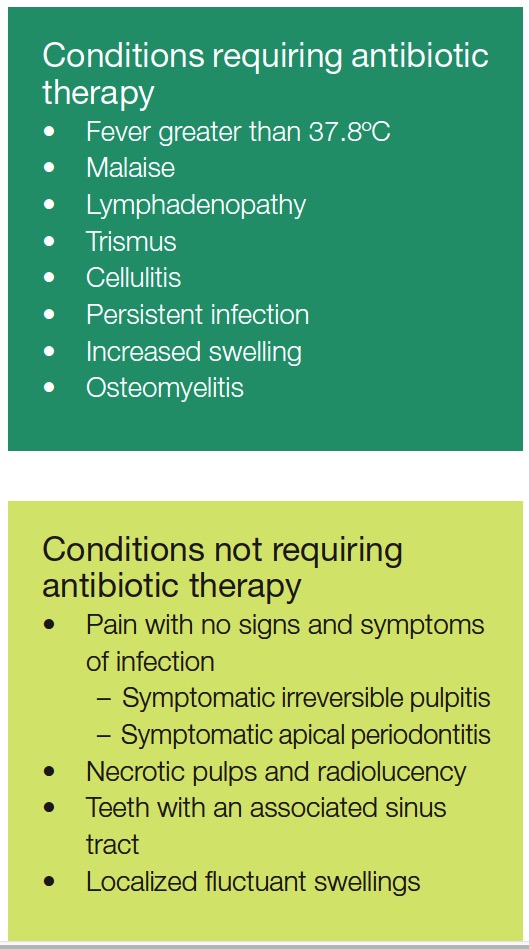
 Never prescribe antibiotics for an irreversible pulpitis. Patients may request that you prescribe. Explain your rationale and advise analgesics only (e.g., ibuprofen), as the pulp is inflamed and not infected. In most cases, the inflammatory process eliminates bacteria that emerge from the apical foramen, and the immune system also stops bacteria spreading into the periapical tissues.
Never prescribe antibiotics for an irreversible pulpitis. Patients may request that you prescribe. Explain your rationale and advise analgesics only (e.g., ibuprofen), as the pulp is inflamed and not infected. In most cases, the inflammatory process eliminates bacteria that emerge from the apical foramen, and the immune system also stops bacteria spreading into the periapical tissues. 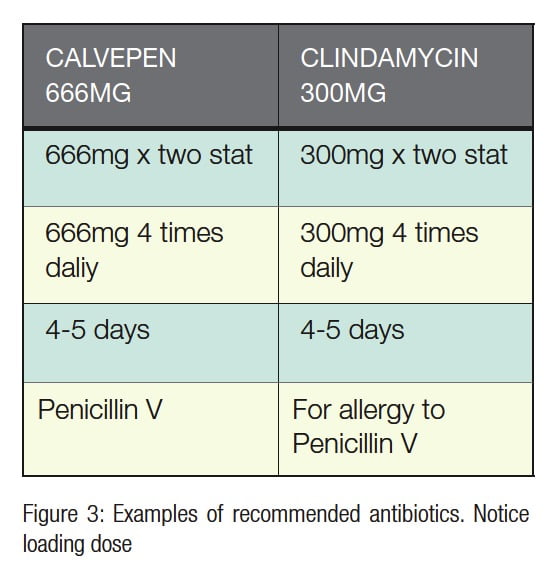 Asymptomatic apical periodontitis does not require antibiotic therapy, and endodontic treatment alone is sufficient (AAE ENDODONTICS: Colleagues for Excellence, 2012).
Asymptomatic apical periodontitis does not require antibiotic therapy, and endodontic treatment alone is sufficient (AAE ENDODONTICS: Colleagues for Excellence, 2012).
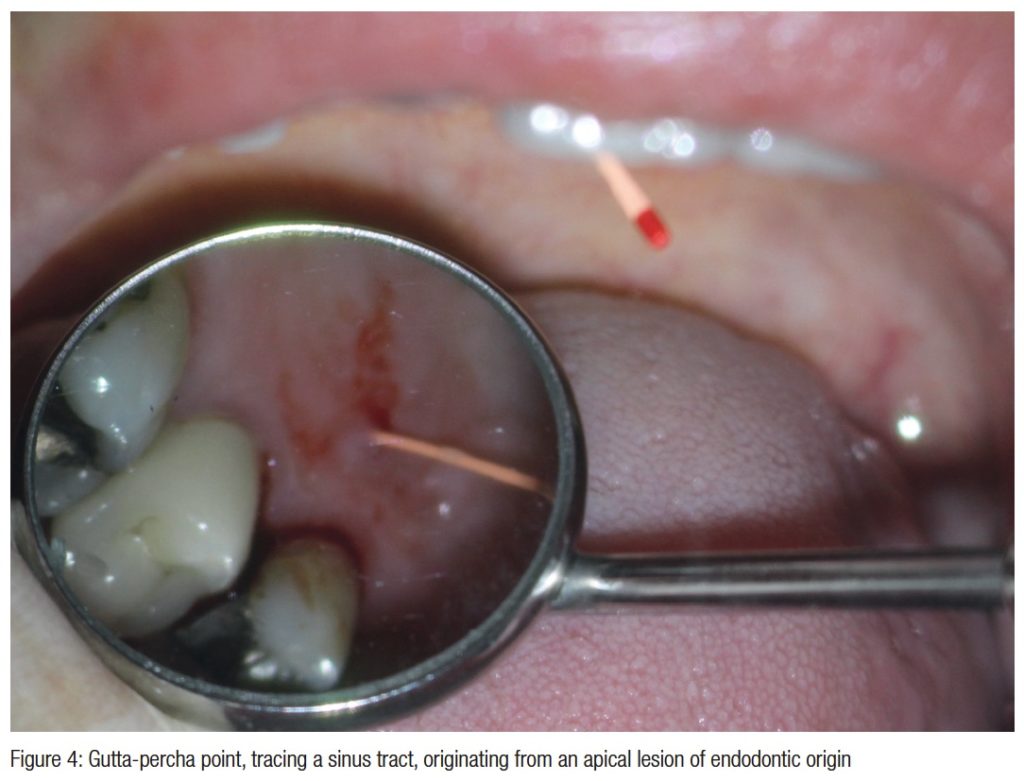
An acute apical abscess can cause a localized fluctuant intraoral swelling with associated pain. Treatment involves endodontic therapy, and there is no need to prescribe antibiotics, but the patient should be advised to take non-steroidal anti-inflammatory drug (NSAID) analgesics such as ibuprofen.
If the intraradicular infection overwhelms the immune system, bacteria will gain access to the periapical tissues. This can result in an acute abscess with a concurrent facial swelling. Endodontic treatment with incision and drainage and antibiotics are indicated in Figure 4.
 In order to prescribe an antibiotic, practitioners need to be aware of their spectrum of activity, so that the antibiotic can target the bacteria, which are responsible for causing endodontic infections. Bacteria that are commonly found in infected root canals are mixed gram positive and gram negative, facultative anaerobes, and strict anaerobes (e.g., Fusobacteria, Prevotella, and Porphyromonas) (Baumgartner, et al., 2008). Penicillin V is the antibiotic of choice, as its spectrum of activity is ideally suited to the bacteria that are found in infected root canals (Baumgartner, Xia, 2003).
In order to prescribe an antibiotic, practitioners need to be aware of their spectrum of activity, so that the antibiotic can target the bacteria, which are responsible for causing endodontic infections. Bacteria that are commonly found in infected root canals are mixed gram positive and gram negative, facultative anaerobes, and strict anaerobes (e.g., Fusobacteria, Prevotella, and Porphyromonas) (Baumgartner, et al., 2008). Penicillin V is the antibiotic of choice, as its spectrum of activity is ideally suited to the bacteria that are found in infected root canals (Baumgartner, Xia, 2003).
 Antibiotics should be prescribed at a high dose over a short period of time. This reduces the chance of selecting drug-resistant bacteria and lowers the risk to the patient with respect to toxicity and allergy (Pallasch, 1994). The initial dose of the antibiotic should always be higher than the maintenance dose.
Antibiotics should be prescribed at a high dose over a short period of time. This reduces the chance of selecting drug-resistant bacteria and lowers the risk to the patient with respect to toxicity and allergy (Pallasch, 1994). The initial dose of the antibiotic should always be higher than the maintenance dose.
A loading dose should always be employed, and it should be twice the maintenance dose (AAE ENDODONTICS: Colleagues for Excellence, 2012). This guarantees rapid and high blood levels of the antibiotic.
Traditionally, metronidazole was pre-scribed for endodontic infections, but its spectrum of activity is limited to anaerobes. Metronidazole should therefore not be prescribed on its own, as it is not effective against facultative anaerobes (Baumgartner, 2003). Erythromycin was also traditionally prescribed for patients who are allergic to penicillin; however, it should never be prescribed, as it is not effective against anaerobic bacteria (Baumgartner, 2006).
4. Multiple antibiotics are superior to a single antibiotic
Multiple antibiotics will guarantee a greater antibiotic spectrum, but this will result in the selection of drug-resistant bacteria (AAE ENDODONTICS: Colleagues for Excellence, 2012). The only indication for combined antibiotics is a severe infection.
5. Bacterial infections need a “complete course” of antibiotic therapy
There is no such thing as a “complete course of antibiotics” (Pallasch, 1994). The only guide is improvement in the patient’s symptoms, and this is based on the effectiveness and duration of antibiotic therapy (Hessen, Kaye, 1989).
Orofacial infections do not rebound, as long as the source of infection has been removed. Endodontic treatment will remove the source of infection. Therefore, once the patient’s swelling has subsided, the course of antibiotics should be stopped.
Alter existing prescribing regime
In summary, we have discussed and dispelled five myths relating to antibiotic therapy. Antibiotic resistance is an ever-increasing issue, and it can only be combated by effective prescribing with reduced abuse of antibiotics.
By analyzing these myths, practitioners can alter their prescribing regime. This alteration, most importantly, can be implemented the next time you think about prescribing an antibiotic for your patients.
References
1. American Association of Endodontists. Use and abuse of antibiotics. Endodontics: Colleagues for Excellence newsletter. Winter 2012. 2. Baumgartner JC. Antibiotics and the treatment of endodontic infections. American Association of Endodontics Colleagues for Excellence newsletter. Summer 2006. 3. Baumgartner JC, Siqueira JF, Sedgley CM, Kishen A. Microbiology of endodontic disease. In: Ingle, JI, Bakland LK, Baumgartner JC, eds. Ingle’s Endodontics 6. People’s Medical Publishing House-USA. 2008:221-308. 4. Baumgartner JC, Xia T. Antibiotic susceptibility of bacteria associated with endodontic abscesses. J Endod. 2003;29(1): 44-47. 5. Hessen MT, Kaye D. Principles of selection and use of antibacterial agents. Infect Dis Clin North Am. 1989;3(3): 479-489. 6. Jungermann GB, Burns K, Nandakumar R, Tolba M, Venezia RA, Fouad AF. Antibiotic resistance in primary and persistent endodontic infections. J Endod. 2011;37(10):1337-1344. 7. Kinnevey PM, Shore AC, Brennan GI, Sullivan DJ, Ehricht R, Monecke S, Coleman DC. Extensive genetic diversity identified among sporadic methicillin-resistant Staphylococcus aureus isolates recovered in Irish hospitals between 2000 and 2012. Antimicrob Agents Chemother. 2014;58(4):1907-1917. 8. Montagner F, Jacinto RC, Correa Signoretti FG, Scheffer de Mattos V, Grecca FS, Gomes BP. Beta-lactamic resistance profiles in Porphyromonas, Prevotella, and Parvimonas species isolated from acute endodontic infections. J Endod. 2014; 40(3): 339-344. 9. Pallasch TJ. Pharmacology of Anxiety, Pain and Infection. In: Ingle JI, Bakland LK, eds. Endodontics. 4th ed. Williams and Wilkins: Malvern, PA, 1994. 10. Sweeney LC, Dave J, Chambers PA, Heritage J. Antibiotic resistance in general dental practice — a cause for concern? J Antimicrob Chemother. 2004;53(4): 567-576.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


