CE Expiration Date: March 10, 2025
CEU (Continuing Education Unit):2 Credit(s)
AGD Code: 070
Educational aims and objectives
This self-instructional course for dentists aims to review the history, basic features, clinical benefits, and future of the dental operating microscope (DOM) as it pertains to endodontic treatment.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Describe a brief history of the microscope within endodontics.
- Identify the various components that make up the microscope.
- Realize how these various components of the microscope impact the field of view and magnification.
- Recognize the additions that can be implemented with the microscope and how these can impact a clinical practice.
- Describe how the microscope improves a clinical practice and the impact it has shown to have on outcomes.
Dr. Andrew R. Steidley offers insights into the history, uses, and benefits of the dental operating microscope (DOM)
Over approximately the past 30 years, the dental operating microscope (DOM) has made a revolutionary impact on the field of endodontics. While the first clinician to experiment with the DOM was Bowles in 1907, earlier forms of the microscope were unable to gain a foothold due to an overall poor ergonomic design.1,2 One of the earliest studied uses of the DOM in endodontics was written by Dr. Howard Selden back in 1989 when he reported its use for calcified canal identification from a nonsurgical perspective.3 In January 1997, the Commission on Dental Accreditation (CODA) agreed to integrate the DOM into the curriculum of endodontic programs nationwide. However, despite this addition, 2 years later Dr. Pete Mines found that only 52% of clinicians had access to and used the DOM. It was clinicians such as Dr. Gary Carr who helped modify the DOM’s design along with promoting its use that really transformed it from a utilization rate of 52% back in 1999, to 90% in 2009, and 96% as of 2018 (Figure 1).4,5,6

There are three major components of the DOM, including the support system, body of the microscope, and the light source. The support system is generally found in three various forms that include a ceiling mount, a wall mount, or a floor mount. The type of mount desired is in large part predicated on the operatory layout, although in general, it is preferred to use either a wall or a ceiling mount system as the fixed position allows easier adjustment and use. The body of the microscope is composed of eyepieces, binoculars, magnification adjusters, and objective lenses (Figure 2). The eyepiece is found in powers of 6.3x, 10x (most used), 12.5x (most used), 16x, and 20x with adjustable rubber cups to allow for glasses and a diopter (-5 to +5) that is used for ocular accommodation. The binoculars allow for adjustment of interpupillary distance, and they should be adjusted until the two divergent circles of light combine. The binoculars have three types of configurations that include straight, inclined, or inclinable and can have short or long focal lengths. The inclinable version is currently more widely utilized at the present time.

Magnification adjusters are available in 3-, 4-, 5-, and 6-steps via either manual or power zoom. The general layout of the internal body follows: two lenses that project down to two separate prism assemblies, which in turn project down to two binocular objectives that funnel down to one monocular objective that finally projects down to the object as beautifully outlined in Dr. Carr’s 2010 paper.2
One of the keys to any DOM’s design is a light path that is in alignment with the visual path, which in turn results in better illumination overall. The light source is commonly either halogen or LED with an internal fan to avoid overheating. Earlier versions often would utilize xenon light sources, although those have become less prevalent with time due to high costs for replacement bulbs and a relatively short life span of the bulbs (~500-700 hrs). The light exits as two separate beams that result in a stereoscopic effect, which is most important for depth of field. LED light sources have a brilliant white light that tend to last longer, require less power, but suddenly fail at the end of their life span. In contrast, halogen light sources tend to create a bright, white, concentrated light that turns more yellow as it ages until it finally expires. A recent 2020 article by Nakira, et al., showed that both the LED and halogen lights within the DOM resulted in similar user fatigue pre- and post-op.7 Despite this study, the LED light source currently seems to be utilized more among clinicians due in large part to better light stability and longer life span as mentioned previously.
How can clinicians accurately determine the total magnification of their DOM, and how that compares to conventional loupes? The answers can be found when evaluating the equation discussed in 2010 by Dr. Carr and Dr. Castellucci. This equation follows: TM = (FLB/FLOL)x EPx MF where TM is total magnification, FLB is the binocular focal length, FLOL is the focal length of the objective lens, EP is the eyepiece, and MF is the magnification factor of the changer.2 A quick analysis of the equation and its ramifications reveals that an increase in the power of the eyepiece, the focal length of the binocular, and the magnification factor of the DOM all result in an increased magnification and a decreased field of view. In contrast, an increase of the focal length of the objective lens results in a magnification decrease and a field-of-view increase. Overall, the main conclusion that can be drawn from this is that every time the magnification increases, the depth of field and illumination decrease.2 When comparing the DOM and loupes, it has been shown that the DOM can improve magnification by a factor of about 4 to 10, depending on the type of microscope and loupes being utilized. This improved visualization was confirmed via a 2002 study, which found that the identification of canal orifices was more likely via DOM use when compared to visualization via loupes.8 In fact, the DOM has been shown to improve a clinician’s ability to distinguish 2 various points by a factor of approximately 33.2
Constant advancements in technology and many new innovations have led to expanded use of the DOM. The use of image capture technology (ICT) and assistant viewing binoculars in combination with the DOM was first described by Dr. Carr in 1992.9 His review of this topic included the science behind the DOM along with potential uses for these new technologies. In 2009, a review of the basic components needed for ICT concluded that it is an ever-changing field with constant advancements and innovations.10 As time has passed, ICT has experienced an increase in quality with a decrease in cost.
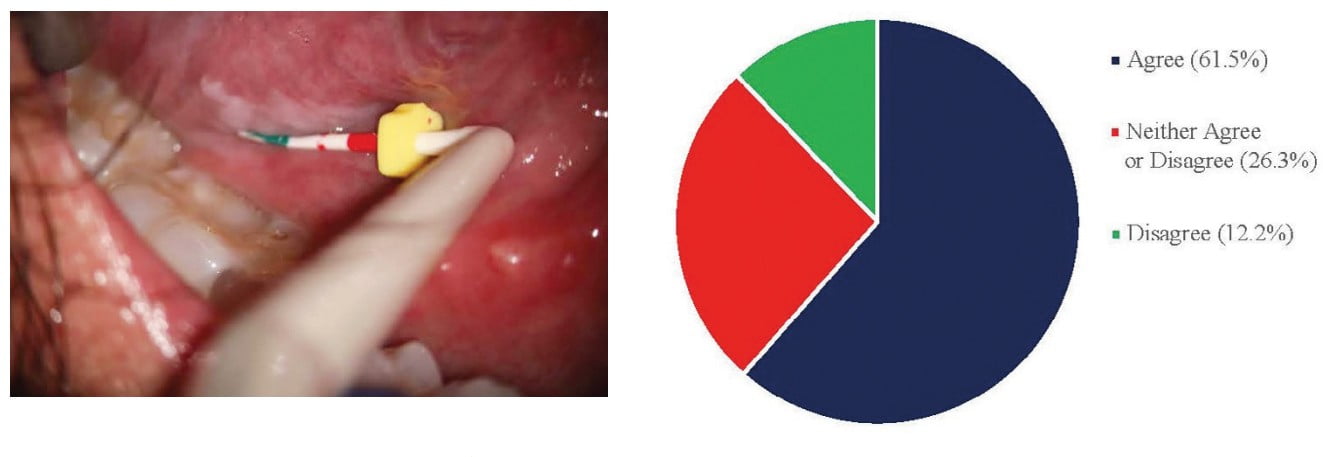
Further, ICT has become directly incorporated into the DOM and is therefore more convenient for clinical use. While the addition of the assistant side scope has been around for many years, it is currently not widely adopted as only 16% of respondents had reported its use in a 2018 study. This same study found that 61% of clinicians currently utilized ICT in some form. Of those that reported its use, 68% of them stated that they used this tool for documentation purposes in general (Figure 3). Overall, most respondents reported that they found the images produced from ICT to be beneficial to their clinical practice (Figure 4).6 While these additions are utilized for slightly different reasons, both will require the addition of a beam splitter to the DOM that allows the light to be shared among the various components.
With an understanding of these components, we can start to evaluate how the DOM has changed practices within the endodontic community. An early study from 2010 demonstrated that while fine motor skills are significantly improved along with improved proficiency, it is an acquired skill that takes time to learn and implement within a clinical practice.11 This steep learning curve likely explains why early on there was resistance to the DOM use, and why CODA’s adoption of the DOM within the endodontic curriculum has been so instrumental in its current acceptance rate of 96%.6 The increased usage of the DOM in the field of endodontics has led to a reported increase in both clinical efficiency and proficiency as well as a decrease in iatrogenic related events.4,5,12 Specifically, the DOM, when utilized nonsurgically, has been shown to provide clinicians with an improved ability to:
- identify cracks (figure 6), extra anatomy (figure 5), and calcified canals
- remove obstructions
- treat dental anomalies
- remove root fillings and sealers
- identify and repair of perforations (Figures 5 and 6)3,13,14
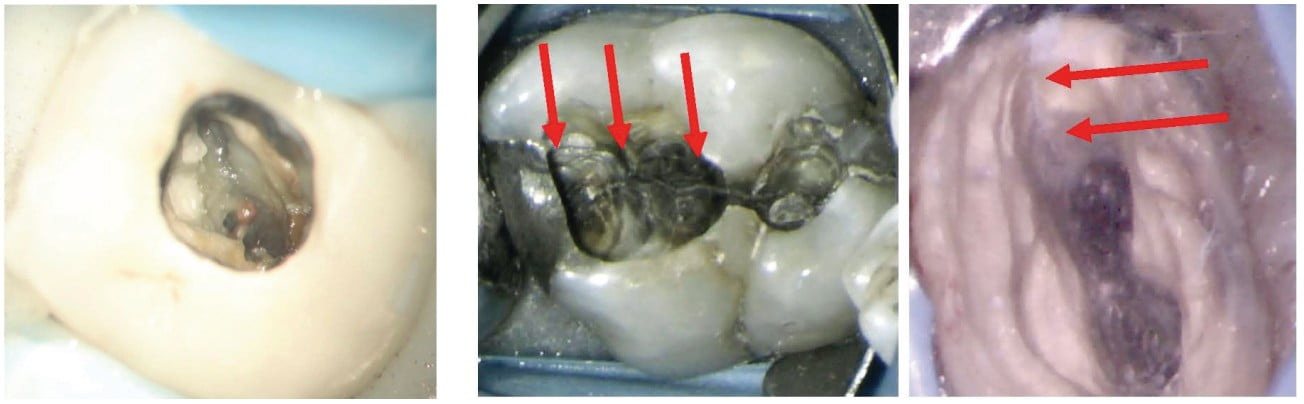
- identify extra canals, cracks, and resorption
- allow improved root end preperarion
- allow improved root endo filling17,18
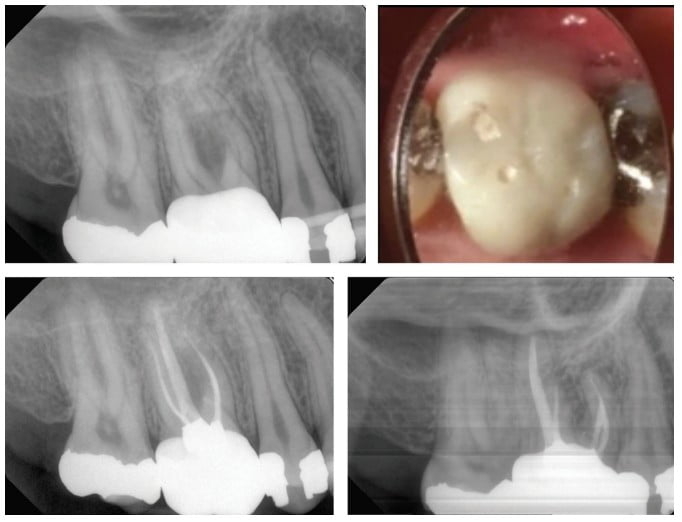
Regarding nonsurgical outcomes, there are precious few studies that truly evaluate success for DOM versus non-DOM treatments. Of those that are present, we discover mixed results. While the Del Fabbro, et al., studies were unable to identify any difference, the Monea, et al., study, completed in 2015, showed a 12% increase in success at 6 months and an 8% increase at 18 months when the DOM was utilized.19,20,21 In contrast, surgical outcomes have a few well publicized meta-analyses from Setzer, et al., that help to illuminate the importance of the DOM. The combined results of these studies showed a success of 59% without magnification, 88% with magnification via loupes, and 94% with magnification via the DOM.22,23 In 2013, Tsesis, et al., found a success rate of 92% when the DOM was utilized, which helped to confirm the importance of the DOM for surgical endodontic outcomes.24
So, what is the future of the DOM within endodontics? I believe it is important to review recent trends in endodontic literature and within the clinical setting, which show a tendency toward more conservative access designs (Figure 7).25,26,27,28 Further, traditional shaping techniques have also started to be replaced by more minimalist designs, especially in the coronal third, to reduce the incidence of fractured teeth. Drs. Khademi and Clark coined the term “pericervical dentin” to illuminate how strategically important dentin is around the cementoenamel junction (CEJ) for the structural stability of a tooth.29 All of these conservative modifications tend to require extra fine motor skills and better visualization, both of which the DOM has shown the ability to improve.2,9,18 Based on this recent shift within the endodontic community toward conservative techniques and studies, which show improved clinical outcomes and an increased prevalence of DOM use overtime, I think it is fair to say that the microscope continues to have a bright future.4,6,19,22,23,24,25,26,27,28,29
Over approximately the past 30 years, the dental operating microscope (DOM) has made a revolutionary impact on the field of endodontics.
Acknowledgments
I want to thank the Fort Bragg endodontic residency, Drs. Jessica Roeber, Claire Anderson, and Thomas Jahnke for their help in obtaining the images shown throughout this article. I also want to extend a special thanks to Drs. Daniel Kersten and Steven Delgado for being the best mentors an endodontist could ask for.
Dr. Aleksander Iofin shows how the dental operating microscope and CBCT can be a “match made in heaven” for endodontic practices. Watch the webinar here: https://endopracticeus.com/webinar/surgical-operating-microscope-and-cone-beam-ct-2/
References
- Bowles SW. A New Adaptation of the Microscope to Dentistry. Dental Cosmos. 1907;49(4):358-362.
- Carr GB, Murgel CA. The use of the operating microscope in endodontics. Dent Clin North Am. 2010;54(2):191-214.
- Selden HS. The role of a dental operating microscope in improved nonsurgical treatment of “calcified” canals. Oral Surg Oral Med Oral Pathol. 1989;68(1):93-98
- Mines P, Loushine RJ, West LA, Liewehr FR, Zadinsky JR. Use of the microscope in endodontics: a report based on a questionnaire. J Endod. 1999;25(11):755-758.
- Kersten DD, Mines P, Sweet M. Use of the microscope in endodontics: results of a questionnaire. J Endod. 2008;34(8):804-807.
- Steidley A, Kersten D, Delgado S, Mines P, Beltran T. Use of the microscope in endodontics: A questionnaire-based study [abstract]. In: J Endod. 2018;44(3): Abstract PR50.
- Nakahira K, Mutoh N, Fuchida S, et al. Effects of different light sources used for dental operating microscope illumination on the visual function of operators. J Oral Biosci. 2020;62(4):363-371.
- Yoshioka T, Kobayashi C, Suda H. High detection rate of root canal orifices under a microscope. J Endod. 2002;28(6):452-453.
- Carr GB. Microscopes in endodontics. J Calif Dent Assoc. 1992;20(11):55-61.
- van As GA. Digital documentation and the dental operating microscope: what you see is what you get. MICRO: The International Journal of MicroDentistry.2009;1(1):30-41.
- Bowers DJ, Glickman GN, Solomon ES, He J. Magnification’s effect on endodontic fine motor skills. J Endod. 2010;36(7):1135-1138.
- AAE Colleagues for Excellence. The Dental Operating Microscope in Endodontics. American Association of Endodontics. Winter 2016.
- Baldassari-Cruz LA, Wilcox LR. Effectiveness of gutta-percha removal with and without the microscope. J Endod. 1999;25(9):627-628.
- Daoudi MF. Microscopic management of endodontic procedural errors: perforation repair. Dent Update. 2001;28(4):176-180.
- Khalighinejad N, Aminoshariae A, Kulid JC, et al. The effect of the dental operating microscope on the outcome of nonsurgical root canal treatment: a retrospective case-control study. J Endod. 2017;43(5):728-732.
- Stropko JJ. Canal morphology of maxillary molars: clinical observations of canal configurations. J Endod. 1999;25(6):446-450.
- Rashad B, Lino Y, Ebihara A, Okiji T. Evaluation of crack formation and propagation with ultrasonic root-end preparation and obturation using a digital microscope and optical coherence tomography. 2019.
- Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32:601-623.
- Monea M, Hantoiu T, Stoica A, Sita D, Sitaru A. The impact of operating microscope on the outcome of endodontic treatment performed by postgraduate students. Eur Sci J. 2015;305-311.
- Del Fabbro M, Taschieri S, Lodi G, Banfi G, Weinstein RL. Magnification devices for endodontic therapy. Cochrane Database Syst Rev. 2009;(3).
- Del Fabbro M, Taschieri S, Lodi G, Banfi G, Weinstein RL. Magnification devices for endodontic therapy. Cochrane Database Syst Rev. 2015;(12).
- Setzer FC, Kohli M, Shah S, Karabucak B, Kim S. Outcome of endodontic surgery: a meta-analysis of the literature — Part 2: Comparison of Endodontic Microsurgical Techniques With and Without the Use of Higher Magnification. J Endod. 2012;38:1-10.
- Setzer FC, Shah S, Kohli M, Karabucak B, Kim S. Outcome Of Endodontic Surgery: A Meta-Analysis Of The Literature – Part 1: Comparison of traditional root- end surgery and endodontic microsurgery. J Endod. 2010;36(11):1757-1765.
- Tsesis I, Rosen E, Taschieri S, et al. Outcomes of surgical endodontic treatment performed by a modern technique: an updated meta-analysis of the literature. J Endod. 2013;39(3):332-339.
- Plotino G, Grande NM, Isufi A, et al. Fracture strength of endodontically treated teeth with different access cavity designs. J Endod. 2017;43(6):995-1000.
- Abou-Elnaga MY, Alkhawas MAM, Kim HC, Refai AS. Effect of truss access and artificial truss restoration on the fracture resistance of endodontically treated mandibular first molars. J Endod. 2019;45(6):813-817.
- Mooktiar H, Hedge V, Srilatha S, Chopra M. Conservative endodontics: a truss access case series. Int J Appl Dent Sci. 2019;5(4):213-218.
- Krishan R, Paque F, Ossareh A, et al. Impacts of conservative endodontic cavity on root canal instrumentation efficacy and resistant to fracture assessed in incisors, premolars, and molars. J Endod. 2014;40(8):1160-1166.
- Clark D, Khademi J. Modern molar endodontic access and directed dentin conservation. Dent Clinic North Amer. 2010;54(2):249-273.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

