CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
The aim of this article is to discuss, with illustrations, the clinical reasons for the cortical window technique in endodontics.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article.
Correctly answering the questions will demonstrate the reader can:
- Realize when and how to perform the cortical window technique.
- Identify the computer-guided cortical window approach.
- Identify the piezotome osteotomy.
- Recognize the advantage of facilitating bone preservation by allowing replacement of the cortical plate.
- Realize the advantage of the 3D-printed guide.
This less invasive approach can be less traumatic and minimize bone loss. See how cortical window access allows for greater efficiency and accuracy for creation of the access window to the roots.
Drs. Naheed Mohamed, Yosi Nahmias, and Ken Serota discuss computer-guided endodontic surgery (CGES) in the last of this 2-part series
Techniques, materials, and innovations in the micro-armamentarium of endodontic microsurgery are seminal to enhanced predictable outcomes by comparison with historical microsurgical procedures.
The superior magnification and illumination of surgical operating microscopes improves the identification of root peripheries, ensures a lesser degree of root reduction, and diminishes the size of osteotomies, thus retaining greater residual bone.
Smaller resection angles (perpendicular to the long axis of the root) reduce the number of tubuli exposed. Lateral canals, canal deltas, isthmus connections, and micro-cracks can be identified prior to root resection, retro-preparation, and retro-sealing.1
Studies of positive treatment outcomes for conventional endodontic surgical therapy show a diverse range of success dependent upon an array of predictors.2,3 A study by Wang, et al., reported an overall healed rate of 74% of assessed teeth; root filling length and size of preoperative lesions proved to be important predictors of treatment outcomes.4
Positive treatment outcomes (94%) were demonstrated by microsurgical techniques.5 Retreatment of failing endodontic procedures demonstrate statistically less positive treatment outcomes than those done by microsurgical techniques (86%); fewer failures ensue.6 These conditions are more readily addressed with microsurgical techniques.7
The computer-guided cortical window approach
A cortical window (bone lid) access to the apical region is less invasive, minimizes bone loss, and is less traumatic in comparison to alternative techniques. The perimeter of the window is determined from radiographs of the area. Radiographs are essential to all aspects of endodontics; however, flat films are two-dimensional images of three-dimensional structures, and so data interpretation is subjective.
Cone beam computed technology (CBCT) enables the clinician to visualize structures in sagittal, axial, and coronal planes. Three-dimensional imaging provides more substantial data for diagnosis, pretreatment planning, posttreatment assessment, and reassessment evaluations.8,9
A printed stereolithographic surgical template can guide the osteotomies during the surgery, minimizing deviation from the digital surgical plan. Surgical templates printed from three-dimensional imaging optimize site preparation, the perimeter of the osteotomy, depth of cortical bone, extent of pathology, and volume of bone graft required.10-13
Piezotome osteotomy
Traditional osteotomies use large, round burs, which remove significant cortical bone. Delayed healing, increased postoperative pain, and other complications may ensue. With microscopes, piezotomes, and ultrasonic tips, a smaller osteotomy is created, thus minimizing the aforementioned sequelae.
Piezo surgery enables micrometric saw cuts, which preserve cortical bone loss and facilitates preservation of root length by lower resection angles and enhanced visibility. In deep spaces, ultrasonic vibrations break down irrigants into small particles readily washed from the crypt.
Less vascular presence in the crypt minimizes use of hemostatic agents (ViscoStat™, Ultradent Products, Inc.) and interference with retro-seal setting time. The use of a piezo surgical device (Figure 1) enables accurate shaping of the cortical window and diminished osseous removal, in contrast to traditional crypt creations that are freehand guided.14

Case report
The patient presented to our surgery with a history of “sporadic discomfort in the gum” overlying tooth LR2. A two-dimensional intraoral radiograph revealed a prior history of root canal therapy and a porcelain-fused-to-metal (PFM) crown (both completed approximately 10 years ago) (Figure 2).
Swelling began the evening prior to the appointment; the patient reported that the throbbing necessitated analgesics for relief of the pain. No sensitivity to pressure or reaction to temperature was noted; the patient could not localize the tooth causing the distress. Treatment options were discussed with the patient; retreatment through the PFM crown was chosen.
Anesthesia was administered (posterior superior alveolar nerve block — 2% xylocaine with epinephrine 1:100,00 and infiltration facially and palatally 2% xylocaine with epinephrine 1:50,000). A conservative access preparation was made; decay was identified proximal to the palatal canal, and no fractures or cracks were noted. Cavit™ (3M) was present beneath the composite core, and the untreated MB2 canal was discovered.15
A reservoir was made in the gutta percha (ProUltra®, Dentsply Sirona ultrasonic tip). Endosolv E (Septodont) was used to soften the gutta percha.16 After debridement and shaping, Ca(OH)2 (UltraCal™ XS, Ultradent) was placed in the root canal space to further enhance disinfection.
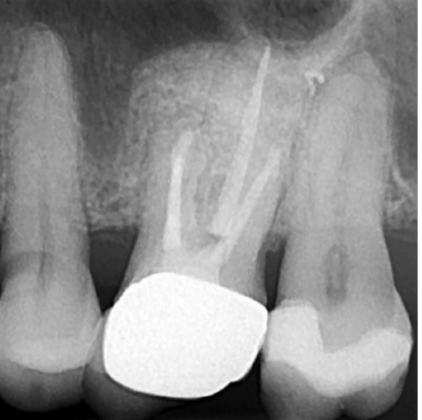
Prior to obturation, drainage was noted coming from the MB2 canal; drainage was arrested, and the canal’s root was filled with vertical condensation of warm gutta percha (VCWG) and AH-Plus® sealer (Figure 3). The patient returned in 6 months for reassessment. Tooth LR2 was within normal limits to percussion, bite, palpation, mobility, and probing.
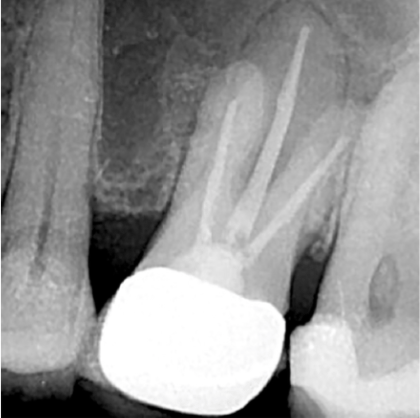
Eighteen months later, the patient returned for a second reassessment appointment (Figure 4). Tooth LR2 was slightly sensitive to percussion, and the overlying gingival tissues were inflamed. The patient was referred for a CBCT; the scan (Figure 5) revealed a common area of rarefying osteitis surrounding the mesial buccal and distal buccal roots, which had caused elevation of the sinus floor. As the endodontic pathology had not resolved, treatment options were proposed. The patient chose to have microsurgical therapy performed.
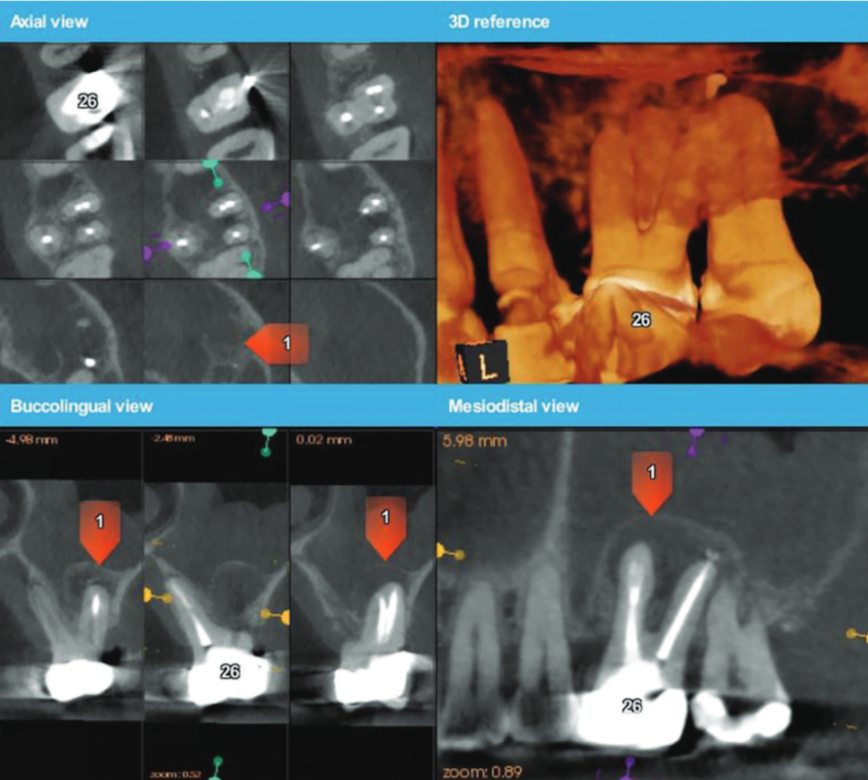
A 3D-printed stereolithographic template was created by combining the CBCT scan data with an intraoral scan’s (3Shape TRIOS® intraoral scanner) digital data. The data was then imported into coDiagnostix® (Dental Wings) software in order to plan our approach and design our cortical window dimensions for optimal access to the roots (Figure 6).
The guided microsurgical approach would facilitate an osteotomy design to minimize the potential for sinus membrane perforation. The 3D-printed guide for the cortical window would guide the length and angle of the osteotomies using the piezosurgical saw (Figure 7).
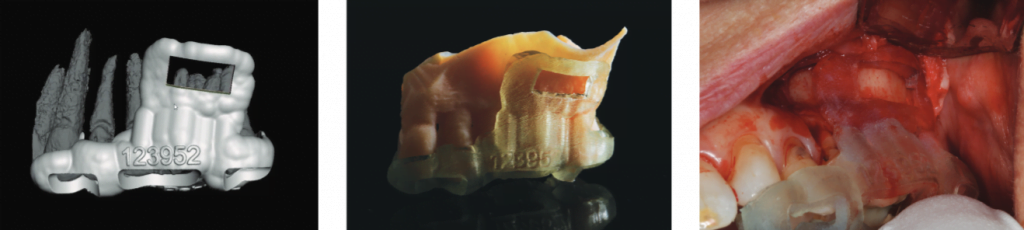

Cervical recession and decay were in evidence about teeth LL1 and LR1 in addition to exposure of the crown margin of tooth LR2. The cervical area of tooth LR3 was severely abraded. An intrasulcular full-thickness muco-periosteal flap was raised; a vertical releasing incision was positioned mesial to tooth LR1.
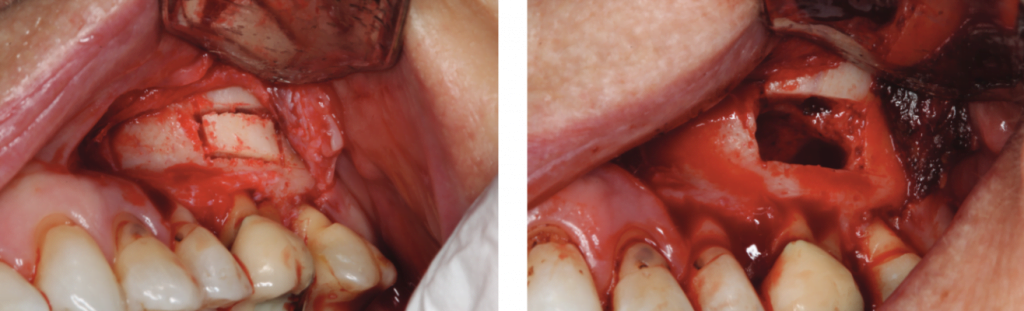
The surgical stent was placed over the maxillary teeth (Figure 8), and a piezotome-guided surgical window was developed using the margins of the stent (Figure 9).

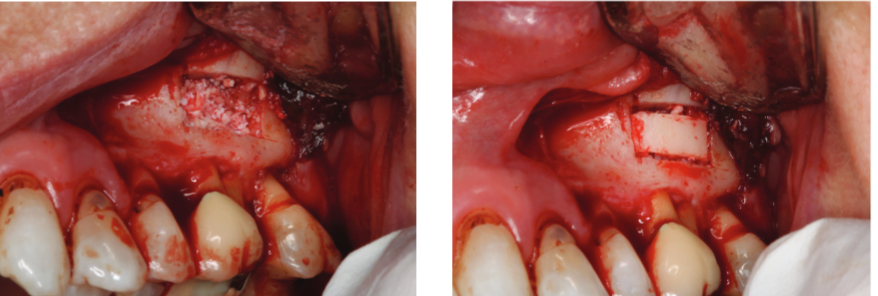
A chisel was used to elevate the cortical plate, and root resection performed with Lindemann burs (Figure 10). The cortical window was placed in sterile saline while the endodontic microsurgery was completed. After resection using Lindemann burs, the root periphery was stained with methylene blue and examined for anomalies, and the root canal space was retro-prepared with ultrasonic tips to a depth of 3 mm, creating a reservoir for the retro-sealing materials.
The retro-preparation was rinsed with ethylenediaminetetraacetic acid (EDTA) and dried with paper points.
Bosworth SuperEBA® was placed (Figure 11) and the root end burnished with a multi-fluted carbide bur. Radiographs were taken at the retro-preparation stage and the retro-sealing stage to ensure accuracy of direction and material placement. The defect was thoroughly debrided and was grafted with allograft (Straumann® AlloGraft) (Figure 12). The cortical bone window was replaced and ensured to have no mobility (Figure 13).
The flap was closed with Ethicon 5-O prolene monofilament sutures (Figure 14), and a postoperative radiograph was taken (Figure 15). The patient was directed to use 800 mg of Advil® and 1000 mg of acetaminophen for pain and to rinse with chlorhexidine.
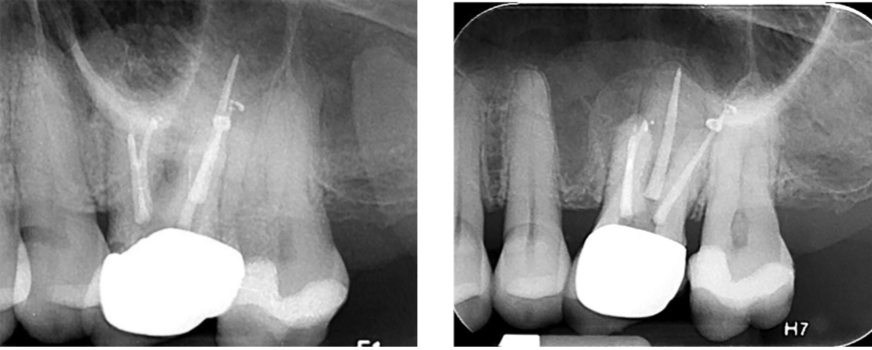
Sutures were removed in 7 days, and the patient was reappointed for reassessment. The re-evaluation radiograph taken at 9 months showed substantial osseous regeneration (Figure 16), and a postoperative CBCT scan was taken after 1 year, showing complete bone regeneration and continuity of the buccal plate. (Figure 17).

Conclusions
Along with surgical operating microscopes and piezotomes, integration of optical scanners and CBCT DICOM files to 3D-printed stereolithographic surgical guides is yet another iteration in the advancement of endodontic microsurgery. This novel, digitally guided approach used in this case report, along with the intraoperative use of a 3D-printed osteotomy guide, allows for greater efficiency and accuracy for creation of the access window to the roots.
The technique gives the advantage of bone preservation by allowing the cortical plate to be replaced, yet still provides adequate access for the apical root preparation. The 3D-printed guide provides a control for the osteotomies without risking damage to vital structures. This digitally guided microsurgical approach provides accuracy, access, control, and bone preservation to the endodontic apical surgery procedure.
As we come upon the dawn of a new age of digital dentistry, we can see the future applications to be endless.
Acknowledgment
Special thanks to Dr. Milan Madhavji of Canaray Oral Radiology for his technical expertise in helping to make our vision a reality.
To review part one of this less invasive approach by Drs. Naheed Mohamed, Yosi Nahmias, and Ken Serota, click here. Subscribers can receive 2 credits after taking the quiz!
References
- Weller RN, Niemczyk SP, Kim S. Incidence and position of the canal isthmus. Part 1. Mesiobuccal root of the maxillary first molar. J Endod. 1995;21(7):380-383.
- Guerreo CG, QuijanoGuaugue S, et al. Predictors of clinical outcomes in endodontic microsurgery: a systematic review and meta-analysis. Giornale Italiano di Endondonzia. 2017;31(1):2-13.
- de Chevigny Dao TT, Basrani B, et al. Treatment outcome in endodontics: the Toronto Study — Phase 4: initial treatment. J Endod. 2008;34(3);258-263.
- Wang N, Knight K, Dao T, Friedman S. Treatment outcome in endodontics: the Toronto Study. Phases I and II: apical surgery. J Endod. 2004;30(11):751-61.
- Tsesis I, Rosen E, Taschieri S, et al. Outcomes of surgical endodontic treatment performed by a modern technique: an updated meta-analysis of the literature. J Endod. 2013;39(3):332-339.
- Setzer FC, Kohli MR, Shah SB, Karabucak B, Kim S. Outcome of endodontic surgery: a meta-analysis of the literature — Part 2: Comparison of endodontic microsurgical techniques with and without the use of higher magnification. J Endod. 2012;38(1):1-10.
- Floratos S, Kim S. Modern Microsurgical Concepts: A Clinical Update. Dent Clin North Am. 2017;61(1):81-91.
- Ahlowalia MS, Patel S, Anwar HM, et al. Accuracy of CBCT for volumetric measurement of simulated periapical lesions. Int Endod J. 2013;46(6):538-546.
- Venskutonis T, Plotino G, Juodzbalys G, et al. The importance of cone-beam computed tomography in the management of endodontic problems: a review of the literature. J Endod. 2014;40(12):1895-1901.
- Kuhl S, Payer M, Zitzmann NU, Lambrecht JT, Filippi A. Technical accuracy of printed surgical templates for guided implant surgery with the coDiagnostiX™ software. Clin Implant Dent Relat Res. 2015;17(suppl 1):e177-e182.
- D’Haese J, Van De Velde T, Komiyama A, Hultin M, De Bruyn H. Accuracy and complications using computer-designed stereolithographic surgical guides for oral rehabilitation by means of dental implants: a review of the literature. Clin Implant Dent Relat Res. 2012;14(3):321-335.
- Pinsky HM, Champleboux G, Sarment DP. Periapical surgery using CAD/CAM guidance: preclinical results. J Endod. 2007;33(2):148-151.
- Strbac GD, Schnappauf A, Giannis K, Moritz A, Ulm C. Guided Modern Endodontic Surgery: A Novel Approach for Guided Osteotomy and Root Resection. J Endod. 2014;43(3):496-501.
- Abella F, de Ribot J, Doria G, Duran-Sindreu F, Roig M. Applications of piezoelectric surgery in endodontic surgery: a literature review. J Endod. 2014;40(3):325-332.
- Stropko JJ, Canal Morphology of maxillary molars: clinical observations of canal configurations. J Endod. 1999;25(6):446-450.
- Hwang JI, Chuang AH, The effectiveness of endodontic solvents to remove endodontic sealers. Mil Med. 2015;180(suppl 3):92-95.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..