CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
This article aims to inform the reader of enterococcus faecalis, a common microorganism associated with endodontic persistence and reinfection.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Realize that fungal-derived silver nanoparticles can be used as root canal disinfectantfor effectively eliminating the resistant microorganisms, including Enterococcus faecalis
(e faecalis) and increase endodontic success rates. - Recognize some characteristics of e faecalis that are integral to finding measures for eradicating this microorganism from root canal infections.
- Recognizing some characteristics of nanoparticles (NPs) for endodontic disinfection.
- Realize some advantages to using fungal-derived silver nanoparticles (AgNPs) as antimicrobial agents.
- Realize the increased stability of nanoparticles.
Editor’s Note: Drs. R. Halkai, K. Halkai, and C. Jyothi show how biosynthesized silver nanoparticles can eradicate e faecalis from infected root canals. Read about the advantages of this type of root canal disinfectant.
Drs. Rahul Halkai, Kiran Halkai, and C. Jyothi investigate a common microorganism associated with endodontic persistence and reinfection
Abstract
Enterococcus faecalis (e faecalis) is a common microorganism associated with endodontic persistence and reinfections. It is one of the most resistant microbes and poses a severe challenge for its eradication during endodontic treatment. Antimicrobial agents in the form of irrigants and intracanal medicaments play an important role in root canal disinfection. Fungal-derived biosynthesized silver nanoparticles (AgNPs), owing to their improved properties — including small size (1-100 nm), eco-friendly, no chemicals used during synthesis, low cost, and broad spectrum of antimicrobial activity without drug resistance — create a new horizon in root canal disinfection. This article highlights the facts of e faecalis related to endodontic infections and the importance of fungal-derived silver nanoparticles as antimicrobial agents in eradicating e faecalis from root canal infections.
Introduction
The main aim of endodontic treatment is to completely eliminate the bacterial infection and prevent reinfection. However, even after contemporary dental surgery with more sophisticated treatment approaches and techniques, we still encounter failures. It is mainly because of the persistence of microorganisms in tissues even after treatment and growing resistance of most of the microorganisms to the available antimicrobial agents (Siqueira, et al., 2010).
Enterococcus faecalis (e faecalis) is a gram-positive, facultative microorganism with pathogenicity ranging from life-threatening diseases in compromised individuals, systemic diseases such as endocarditis, brain abscesses, septicemia, to root canal infections of obturated root canals with chronic apical periodontitis (Halkai, et al., 2012). It occurs in both asymptomatic endodontic primary infections and persistent infections with a high prevalence in secondary infections — 24%-77% (Stuart, et al., 2006). It is also detected from periodontal pockets of refractory periodontal diseases. Culture-based studies have reported e faecalis with a 1%-5% prevalence rate in subgingival sites of periodontitis cases where as PCR evaluation showed 48% (Ram, et al., 1992; Colombo, et al., 2013).
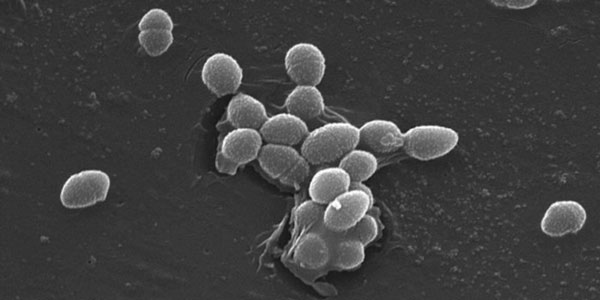
E faecalis possesses several virulence factors such as cytolysis, lytic enzymes, aggregation substances (AS), pheromones, surface proteins, and lipotechoic acid (Halkai, et al., 2012). It produces collagen-binding proteins such as angiotensin-converting enzyme (ACE) and serine protease and gelatinase, which helps for bacterial adhesion and penetration even under stress (Halkai, et al., 2013). ACE promotes resistance to killing by human neutrophils. E faecalis invades dentinal tubules and root cementum probably through lipoteichoic acids (LTA), and to the collagen through aggregation substance (AS) and other surface adhesins (Halkai, et al., 2013). It gets attached to protruding obturating material in periapical tissues (Nair, 2004). E faecalis can penetrate into root cementum even after obturation (Halkai, et al., 2014). Possession of these virulence factors might be an advantage over other species for its surveillance in infected root canals (Halkai, et al., 2013). It can grow at 10°C and 45°C, at pH 9.6, in 6.5% NaCl broth, and survive at 60°C for 30 minutes. It can withstand harsh environmental conditions. It can adapt to adverse conditions such as after pre-exposure to sublethal stress conditions. It becomes less sensitive to normal lethal levels of sodium dodecyl sulfate, bile salts, hyperosmolarity, heat, ethanol, hydrogen peroxide, acidity, and alkalinity (Halkai, et al., 2012). E faecalis cells remain viable for extended periods even in starvation and become resistant to UV irradiation, heat, sodium hypochlorite, hydrogen peroxide, ethanol, and acid (Giard, et al., 1996). E faecalis, moreover, can enter the viable but non-cultivable (VBNC) state — a survival mechanism adopted by a group of bacteria when exposed to environmental stress and resuscitate upon returning to favorable conditions (Lleò, et al., 2001). VBNC e faecalis adapts to cell wall alterations that might protect the microbe even in stressful conditions and it can adhere to the root canal surfaces (Signoretto, 2000). The ability of e faecalis to tolerate or adapt to harsh environmental conditions is an advantage over other species. Hence, it can survive in root canal infections, where nutrients are scarce, and there are limited means of escape from root canal medicaments (Halkai, et al., 2012). E faecalis resists root canal treatment procedures and can penetrate into dentinal tubules and survive even in nutrition-depleted conditions of obturated canals leading to persistent or reinfections (Halkai, et al., 2014; Halkai, et al., 2016). It forms communities organized in biofilm, which resist the destruction by enabling the bacteria to become 1,000 times more resistant to phagocytosis, antibodies, and antimicrobials than non-biofilm-producing organisms (Halkai, et al., 2018). Along with instrumentation, thorough root canal disinfection as well as irrigation and intracanal medication play an important role in bacterial elimination (Halkai, et al., 2018). Several root canal disinfectants with effective antimicrobial efficacy have been used; however, it is still difficult to eradicate e faecalis completely from root canal system. It is resistant to most of the antimicrobial agents and exhibit intrinsic resistant mechanisms. It harbors antibiotic-resistant determinants carried on transferable plasmids (Sedgley, 2005). E faecalis has the ability to invade dentinal tubules and provides protection from chemomechanical preparation and intracanal medicaments. It even resists the high pH of calcium hydroxide (Halkai, et al., 2012). E faecalis binds to collagen even in the presence of serum. It congregates with other bacterial species and survives in root canals in the form of multispecies biofilms, and as the e faecalis biofilms mature on root surfaces, it calcifies for more stability (Johnson, et al., 2006). A few studies suggest e faecalis penetrates and adheres into root cementum and can entomb into the critical areas beyond minor constriction even after three-dimensional obturation (Halkai, et al., 2014; Halkai, et al., 2016). E faecalis colonies were found under confocal laser-scanning microscope and confirmed by PCR techniques and suggested MTA sealing in apical 1 mm-2 mm before obturation, as an effective way to prevent e faecalis colonization (Halkai, et al., 2016). However, through this approach, periapical lesions can be prevented, but concern is raised regarding the entombed e faecalis in dentinal tubules, lateral canals, irregularities of root canal, which harbors e faecalis even after thorough root canal instrumentation, root canal disinfection, and 3D obturation and also with MTA apical sealing as suggested. Therefore, to overcome this, newer antimicrobial agents with increased antimicrobial activity without causing drug resistance have to be developed to completely eliminate such resistant microbes for successful endodontic treatment outcomes (del Pozo, 2007).
Introduction of nanoparticles
Recently nanoparticles (NPs) have gained popularity for endodontic disinfection. They are particles in the size range of 1-100 nm. Due to the extremely small size, they penetrate the deeper tissues and exhibit potent antimicrobial activity (Halkai, et al., 2016). Due to growing concern about various chemicals used during synthesis and causing hazards to human health and environment, there is shift to biological approaches for production of nanoparticles. Among the several nanoparticles, silver nanoparticles (AgNPs) exhibit a broad spectrum of activity and are biocompatible (Halkai, et al., 2017). AgNPs can be produced using several biological vectors such as bacteria, fungi, yeasts, leaf extracts, roots, bark, and so on. Biosynthesized AgNPs have emerged as novel antimicrobial agents with efficient antimicrobial activity against several pathogens (Halkai, et al., 2018; Halkai, et al., 2017; Ninganagouda, et al., 2013), but among these, fungal-derived AgNPs are widely used in medical field. They possess a broad spectrum of activity and are biocompatible (Halkai, et al., 2017).
Advantages of fungi include the following:
- They require simple media to grow.
- They are not technique-sensitive.
- They can be produced on large scale.
- They are low cost.
- They do not require toxic chemicals in synthesis and are therefore considered as naturally occurring nanofactories (Halkai, et al., 2016).
Benefits
Fungal-derived AgNPs act synergistically in distinct targets, and there is no interference with antimicrobial resistance mechanisms. AgNPs can penetrate the tissues owing to their extremely small size and high-surface area; hence, their potential use for resistant microbes (Sapra, et al., 2014). Recent studies have shown application of fungal-derived AgNPs against gram-positive, gram-negative, and multi-drug resistant (MDR) strains (Halkai, et al., 2017; Ninganagouda, et al., 2013). They exhibit potent antimicrobial activity against the resistant endodontic pathogen e faecalis even in biofilm form (Halkai, et al., 2018; Halkai, et al., 2017; Halkai, et al., 2018).
Fungal-derived AgNPs were also employed as antimicrobial agents against endo-perio pathogens such as e gingivalis, e faecalis, and bacillus pumilus with effective antimicrobial efficacy (Halkai, et al., 2017; Halkai, et al., 2018). E gingivalis is one of the most common microorganisms associated with different types of periodontal diseases and is also seen with primary endodontic infections, periapical diseases, and endo-perio lesions (Halkai, et al., 2017). Recent studies show effective antimicrobial efficacy of fungal-derived AgNPs against p gingivalis (Halkai, et al., 2017; Halkai, et al., 2018; Halkai, et al., 2017). Bahadoor, et al., (2013) incorporated fungal-derived AgNPs in MTA (Nanosilver MTA) and evaluated antimicrobial efficacy against p gingivalis by the agar diffusion method. NSMTA exhibited effective antimicrobial efficacy with 9 mm-13 mm zones of inhibition against p gingivalis (Bahadoor, et al., 2013).

More benefits
It is shown that fungal-derived AgNPs are biocompatible and exhibit least cytotoxicity. Han, et al., (2014) reported cytotoxicity of biosynthesized AgNPs on human lung epithelial adenocarcinoma cell lines with IC50 values of 20µg/ml compared to the synthetic AgNPs with IC50 values of 70µg/ml, indicating biosynthesized AgNPs are more effective at a minimum dose compared to synthetic AgNPs. Halkai, et al., (2016) reported at a minimum concentration of 8 µg/ml. The percentage inhibition of fungal-derived AgNPs against human gingival fibroblasts (HGF) cell line by MTT assay was found to be 2.11%. A maximum cytotoxicity of 65.24% was found at the concentration of 512 µg/ml. At concentration 256 µg/ml AgNPs exhibited 46.36% inhibition. Some 50% inhibition (CTC50) was found at a concentration of 260 µg/ml. A concentration less than 260 µg/ml will be effective against diseased cells and is safe for the healthy cells (Halkai, et al., 2017). Therefore, biosynthesized AgNPs can be considered for endodontic applications as well; however, further studies need to be done before confident use of these particles.
Increased stability of nanoparticles
Sapra, et al., (2014) described the advantages of nanoparticles such as they possess increased stability, controlled release rate, and high dispersibility in aqueous medium. Owing to their small size, NPs can penetrate deeper regions that may be inaccessible to other drugs. Therefore, the clinician should reduce the frequency of administration and provide uniform distribution of active agents (Sapra, et al., 2014). Since e faecalis is associated with different types of infections, it is recommended that fungal-derived AgNPs can be used as an alternative to the root canal irrigants or ICMs; they can be incorporated with other ICMs such as calcium hydroxide for synergetic action and can be used as drug delivery systems during periodontal therapy (Halkai, et al., 2016). They can be effectively used as antimicrobial agents during management of endo-perio lesions eradicating the different types of microorganisms including e faecalis.
Conclusion
Due to complexity of the root canal morphology and persistence of endodontic infections, as well as e faecalis being the dominant microorganism associated with persistent and re-infections, fungal-derived AgNPs show a new horizon with their properties as a root canal disinfectant for effectively eliminating the resistant microorganisms, including e faecalis and increasing the endodontic success rate. However, further in vivo, in vitro studies must be done for effective use of these particles in clinical use.
After reading about how biosynthesized silver nanoparticles can eradicate e faecalis from infected root canals, check out this article about how other endodontic specialists identified and resolved edema of the head and neck in a 7-year-old. https://endopracticeus.com/clinical-articles/identification-resolution-edema-head-neck-7-year-old-child/
References
- Ansari MA, Khan HM, Khan AA, et al. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol Med. 2011;3(2):141-146.
- Bahadoor A, Esmaeili D, Khaledi A, Ghorbanzadeh R. An in vitro assessment of the antibacterial properties of nanosilver Iranian MTA against Porphyromonas gingivalis. J Chem Pharm Res. 2013;5:65‑71.
- Colombo AV, Barbosa GM, Higashi D, et al. Quantitative detection of Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa in human oral epithelial cells from subjects with periodontitis and periodontal health. J Med Microbiol. 2013;62(P10):1592-1600.
- del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82(2):204-209.
- Giard JC, Hartke A, Flahaut S, et al. Starvation-induced multi-resistance in Enterococcus faecalis JH2-2. Curr Microbiol. 1996;32(5):264-271.
- Han JW, Gurunathan S, Jeong JK, et al. Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res Lett. 2014;9(1):459.
- Halkai R, Hegde MN, Halkai K. Enterococcus faecalis can survive extreme challenges – an overview. NUJHS. 2013;2(3):49-53.
- Halkai R, Hegde MN, Halkai K. Enterococcus faecalis cause for persisting infection a confocal analysis. NUJHS. 2013;3:67-72.
- Halkai R, Hegde MN, Halkai K. Evaluation of the presence of Enterococcus faecalis in root cementum: A confocal laser scanning microscope analysis. J Conserv Dent. 2014;17(2):119-123.
- Halkai RS, Hegde MN, Halkai KR. Evaluation of Enterococcus faecalis adhesion, penetration, and method to prevent the penetration of Enterococcus faecalis into root cementum: Confocal laser scanning microscope and scanning electron microscope analysis. J Conserv Dent. 2016;19(6):541-548.
- Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai RS. Evaluation of antibacterial efficacy of biosynthesized silver nanoparticles derived from fungi against endo-perio pathogens Porphyromonas gingivalis, bacillus pumilus, and Enterococcus faecalis. J Conserv Dent. 2017;20(6):398-404.
- Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Antibacterial efficacy of biosynthesized silver nanoparticles against Enterococcus faecalis biofilm: An in vitro study. Contemp Clin Dent. 2018;9(2):237-241.
- Halkai R, Hegde MN, Halkai K. Root cementum invasion and adhesion by Enterococcus faecalis confocal analysis. NUJHS. 2012;2:44-49.
- Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Biosynthesized silver nanoparticles from fungi as antimicrobial agents for endo-perio lesions – a review. Annual Res Rev Bio. 2016;10(6):1-7.
- Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Evaluation of antibacterial efficacy of fungal-derived silver nanoparticles against Enterococcus faecalis. Contemp Clin Dent. 2018;9:45-48.
- Halkai KR, Halkai R, Mudda JA, Shivanna V, Rathod V. Antibiofilm efficacy of biosynthesized silver nanoparticles against endodontic-periodontal pathogens: An in vitro study. J Conserv Dent. 2018;21(6): 662-666.
- Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai RS. Biosynthesis, characterization and antibacterial efficacy of silver nanoparticles derived from endophytic fungi against P. Gingivalis. J Clin Diagn Res. 2017; 11(9):ZC92-ZC96.
- Johnson EM, Flannagan SE, Sedgley CM. Coaggregation interactions between oral and endodontic Enterococcus faecalis and bacterial species isolated from persistent apical periodontics. J Endod. 2006;32(10):946-950.
- Lleò MM, Bonato B, Tafi MC, et al. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol. 2001;91(6):1095-1102
- Nair PNR. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15(6):348‑381
- Ninganagouda S, Rathod V, Jyoti H, Singh D. Extracellular biosynthesis of silver nanoparticles using Aspergillus flavus and their antimicrobial activity against gram negative MDR strains. Int J Pharma Bio Sci. 2013;4(4):222-229.
- Rams TE, Feik D, Young V, Hammond BF, Slots J. Enterococci in human periodontitis. Oral Microbiol Immunol. 1992;7(4):249-252.
- Sapra P, Patel BD, Patel DV, Borkhataria CH. Review: Recent advances in periodontal formulations. Int J Pharm Chem Anal. 2014;1:65‑74.
- Sedgley CM, Molandar A, Flannagan SE, et al. Virulence, phenotype and genotype characteristics of endodontic Enterococcus spp. Oral Microbiol Immunol. 2005;20(1):10-19.
- Signoretto C, Lleò MM, Tafi MC, Canepari P. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl Environ Microbiol. 2000;66(5):1953-1959.
- Siqueira JF Jr, Rôças IN, Ricucci D. Biofilms in endodontic infection. Endodontic Topics. 2010;22(1):33-49.
- Stuart CH, Schwartz SA, Beeson TJ Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32(2):93-98.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..


