Drs. Tiago André Fontoura de Melo, Karen Barea de Paula, Francisco Montagner, Alcione Luiz Scur, Liviu Steier, Roberta Kochenborger Scarparo, José Antônio Poli de Figueiredo, and Fabiana Vieira Vier-Pelissera present a study into endodontic disinfection effectiveness
Abstract
The present study evaluated the effect of ozone gas (OZY® system) and high-frequency electrical pulses (Endox® system), over Enterococcus faecalis cells. Forty plastics tubes were filled with 30 µL of E. faecalis (ATCC 29212) suspension and were divided into four experimental groups, according to the disinfection. Protocol: Group 1 — E. faecalis suspension (no disinfection protocol) (Control Group); Group 2 — suspension + OZY® system, a pulse-120-second (Endox®); Group 3 — suspension + OZY® system, four 24-second pulses (Endox®); Group 4 — suspension + Endox® system alone as disinfection. After each specific disinfection protocol, 20 µL of contaminated BHI broth was analyzed for CFU counting. In the first analysis (M1), 10 µL of contaminated BHI was plated over BHI agar and incubated at 37ºC for 24 hours. The other 10 µL remaining was placed into a plastic tube and incubated for 7 days. Then an aliquot of the broth was placed on agar, and CFU counting was also performed (M2). Statistical analysis was carried out. The significance level was set at 5%. The disinfection protocols employed were not able to inactivate the E. faecalis strains in both periods of analysis. The use of ozone gas or high-frequency electric pulses was not effective to reduce the E. faecalis load.
 Introduction
Introduction
The failure of endodontic treatment can be associated with disinfecting procedures performed within the root canal that do not effectively promote the control and elimination of infection (Lima, et al., 2015). Some microorganisms, such as E. faecalis can remain viable within the root canal system, even after completion of the mechanical chemical preparation (Siqueira, et al., 2000) and the use of an intracanal medication and irrigation for canal disinfection (Evans, et al., 2002).
E. faecalis has inherent antimicrobial resistance, the ability to adapt to harsh environmental changes, and the ability to invade into the dentinal tubules. Therefore, they remain protected and are difficult to eliminate (Zhang, et al., 2015). They are facultative anaerobes, possessing the ability to grow in the presence or absence of oxygen (Rôças, et al., 2004). E. faecalis overcomes the challenges of survival within the root canal system in several ways. It has the capacity to endure prolonged periods of starvation until an adequate nutritional supply becomes available (Figdor, et al., 2003). E. faecalis is able to survive in areas of high pH produced by Ca(OH)2, due to a proton-pump mechanism (Evans, et al., 2002). According to Vianna, et al. (2009), some bacteria may proliferate in pH around 7 and that an increase or decrease of pH can cause reversible and temporary inactivation. However, once re-established to the ideal pH, it returns to its metabolic activities. So far, some substances and intracanal medication can act over the E. faecalis in reversible form for a certain period, but not kill it.
The ozone in both gaseous and aqueous states is a potent oxidizing agent with antimicrobial effects, already described in the literature (Nagayoshi, et al., 2004). The oxidation of the bacterial cell wall and cytoplasmic membrane affects the osmotic balance, which may result in cell lysis (Bünning, Hempel, 1996). Likewise, the use of high-frequency electrical pulses system, which applies high-frequency electrical currents, has shown satisfactory results over the microbial membrane (Cassanelli, et al., 2008; Aranda-Garcia, et al., 2012). However, it has not been elucidated if they can exert an antimicrobial effect that remains over time.
The aim of this study was to evaluate in vitro the effect of ozone gas and high- frequency electrical pulses, over E. faecalis strains in a liquid suspension, as occurs into the main root canal, and to determine if there was a synergetic effect when both disinfection methods were used together and improved bacterial kill resulted.
Materials and methods
Sample preparation and contamination
Forty plastic tubes (Kasvi, Curitiba, Paraná, Brazil) were previously sterilized in an autoclave (Cristófoli, Curitiba, Paraná, Brazil) for a period of 30 minutes. The strain used in the study was of E. faecalis ATCC 29212.
Bacteria grew on a medium of broth BHI (Brain Heart Infusion) for a period of 24 hours at 37°C and 10% CO2 atmosphere in a bacteriological incubator.
The number of colony-forming units (CFU/g/mL) on suspension was determined by plate count agar containing blood. The suspension of E. faecalis was diluted up to 10-8 in 0.85% saline and 100 µL of 10-6, 10-7, and 10-8 suspension concentration. The strains were seeded on blood agar plates in duplicate, using a sterile Drigalsky rod, and plates were incubated at 37°C for 24 hours. After this period, a count of CFU/mL of boards was performed in order to check the growth of 15 to 150 colonies. Bacterial density ranged from 4.0 x 108 to 7.2 x 108.
The tubes were filled with 30 µL of the E. faecalis. The forty Eppendorfs were randomly divided in four experimental groups (Table 1).
Disinfection procedure
Disinfection protocols performed in each experimental group were:
- Group 1 — Positive Control: the E. faecalis contaminated Eppendorfs did not undergo any disinfection protocol.
- Group 2 — OZY® system/1 pulse: the tip of the OZY® system was introduced inside the Eppendorfs, just one 120-second-long pulse was delivered, as described by Kustarsi, et al., (2009).
- Group 3 — OZY® system/4 pulses: the OZY® system was introduced inside the s, and four 24-second pulses each were delivered, according to Case, et al. (2012). There was a 5 second interval between pulses.
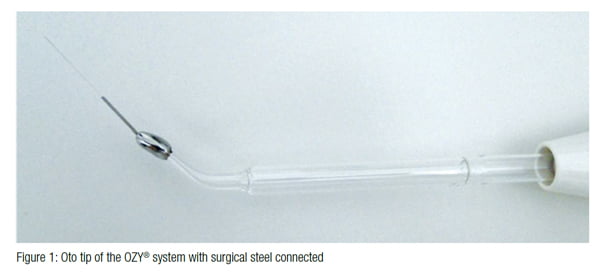
Due to the unavailability of a specific tip to use with the OZY® system (Endox SRL, Italy) for root canal treatment, a device was developed for the “Oto” tip. This adapter was made of 420 surgical steel with 0.20 mm diameter at the end and 30 mm length (Figure 1).
 The OZY® system was operated with 5 N intensity, according to the manufacturer’s instructions. The only variation was in the device activation time between the OZY® 1 pulse and OZY® 4 pulse groups.
The OZY® system was operated with 5 N intensity, according to the manufacturer’s instructions. The only variation was in the device activation time between the OZY® 1 pulse and OZY® 4 pulse groups.
- Group 4 — Endox®: the protocol for the use of Endox® system (Lysis srl, Milan, Italy) (Figure 2) employed in the experiment was the same as described by Lendini, et al., (2005).
The device black probe (measuring 30 mm in length and 0.20 mm in diameter) was introduced inside the Eppendorfs. Four 600 kHz pulses were delivered inside each tube with a 1/10 of a second standard time for each application.
Microbial counts analysis
After performing disinfection protocols inside the laminar flow chamber, 20 µL of BHI broth contaminated with E. faecalis were collected with the aid of a micropipette. At first analysis (M1), 10 µL of contaminated BHI were plated in Agar BHI and incubated at 37ºC for 24 hours. The remaining 10 µL were placed in a plastic tube and incubated for 7 days. Then the inoculated agar plates were analyzed (M2). The CFU values were recorded and inserted into a spreadsheet.
Statistical analysis
Comparisons between groups and moments were performed using non-parametric tests (Wilcoxon T, Kruskal-Wallis H, and Mann-Whitney U for post hoc procedures). No adjustments were made for multiple comparisons. Significance level was set at α = 0.05. Analyses were conducted using SPSS version 22.0 (SPSS Inc, Chicago, Illinois).
Results
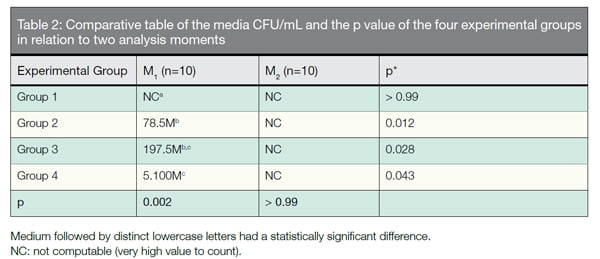
The results are expressed in Table 2. There was no statistical difference between the media of CFU/mL disinfection protocols employed. None of the disinfection methods was effective to reduce the E. faecalis
CFU count.
 Discussion
Discussion
The complete eradication of micro-organisms through the endodontic treatment protocols is difficult (Atila-Pektaş, et al., 2013). A possible justification for this difficulty up until this point is that many of the therapeutic ways to disinfect are unable to inactivate the active power of bacterial strains.
 Vianna, et al., (2009) and Mehrvarzfar, et al., (2011) reported that after a few hours of the action of different substances, there had been growth of E. faecalis strains. The tested disinfection protocols only promoted a reversible inactivation of bacteria. They were not able to completely disrupt the E. faecalis cells.
Vianna, et al., (2009) and Mehrvarzfar, et al., (2011) reported that after a few hours of the action of different substances, there had been growth of E. faecalis strains. The tested disinfection protocols only promoted a reversible inactivation of bacteria. They were not able to completely disrupt the E. faecalis cells.
In the present study, E. faecalis was chosen as the test microorganism because it is one of the most resistant microorganisms found in infected root canals (Sedgley, et al., 2008), and it has been reported that cases with refractory endodontic treatment were associated with this bacterial strain (Stuart, et al., 2006).
The analysis after performing disinfection protocols demonstrated a significant reduction in the level of CFU using an ozone gas system in relation to the high-frequency electrical pulses. This finding is consistent with the study by Virtej, et al., (2007) in which the Endox® system presented a less antimicrobial effect than sodium hypochlorite, BioPure MTAD®, and the ozone gas HealOzone® system.
Although some studies, such as Cassanelli, et al. (2004), Hubbezoglu, et al. (2014), and Kaptan, et al. (2014), have demonstrated a bactericidal effect of ozone gas and high-frequency electrical pulses equipment, none of the disinfection protocols carried out in the study promoted a significant elimination of the E. faecalis cells. According to Virtej, et al., (2007), Kustarci, et al. (2009), Karale, et al. (2011), and Zan, et al. (2013), the antibacterial effect of the aqueous ozone and high-frequency electrical pulses was insufficient when compared with the sodium hypochlorite solution. In addition, in the second period (M2), 7 days after collection, there was an increase in the CFU counts when compared to the control group and immediately after the disinfection protocols.
The study of Virtej, et al. (2007), observed bacterial growth after a week of incubation. It reaffirms that both protocols of disinfection were able to inactivate the bacteria but only for a short period of time. Once re-established, the environmental condition, microorganisms, and metabolic activities returned normally.
Data obtained in the study of Aranda-Garcia, et al. (2012), are in accordance with the considerations reported in this experiment. The authors compared the effectiveness of antibacterial Endox Plus® system and the 2.5% sodium hypochlorite solution in combination with BioPure MTAD® or EDTA on root canals infected with E. faecalis. Although the data have initially been satisfactory, all disinfection procedures allowed the bacterial recovery 7 days after treatment. It demonstrated the persistence and viability of bacteria inside the root canal system. Polydorou, et al. (2006), observed bacterial growth 8 weeks after the use of the HealOzone® system.
According to Cassanelli, et al. (2008), the high-frequency electrical pulses system induces pore formation and other defects in the membrane of the exposed bacteria. Nagayoshi, et al. (2004), noted that ozone gas has the power to damage the bacterial membrane by tissue oxidation, thus increasing its permeability. Therefore, both systems may exert reversible inactivation on the bacterial cell membrane. However, the restoration of bacterial environment and substrate may have promoted the return of their physiological conditions.
The results obtained in the study reinforce the importance of finding new alternatives for greater effectiveness in the process of endodontic disinfection. Perhaps, one of the possibilities would be the association of irrigating solutions with the tested equipment because the damage to the bacterial membrane promoted by them can facilitate and potentiate the antimicrobial action.
According to the results of the present study, both ozone gas and the high-frequency electrical pulses were not effective to eliminate E. faecalis, immediately after the disinfection protocol and after 7 days.
- Aranda-Garcia AR, Guerreiro-Tanomaru JM, Faria-Júnior NB, et al. Antibacterial effectiveness of several irrigating solutions and the Endox Plus system – an ex vivo study. Int Endod J. 2012;45(12):1091-1096.
- Atila-Pektaş B, Yurdakul P, Gülmez D, Görduysus O. Antimicrobial effects of root canal medicaments against Enterococcus faecalis and Streptococcus mutans. Int Endod J. 2013;46(5):413-418.
- Bünning G, Hempel DC. Vital-fluorochromization of microorganisms using 3’, 6’-diacetylfluorescein to determine damages of cell membranes and loss of metabolic activity by ozonation. Ozone Sci Engl. 1996;18:173-181.
- Case PD, Bird PS, Kahler WA, George R, Walsh LJ. Treatment of root canal biofilms of Enterococcus faecalis with ozone gas and passive ultrasound activation. J Endod. 2012;38(4):523-526.
- Cassanelli C, Marchese A, Cagnacci S, Debbia EA. Alteration of membrane permeability of bacteria and yeast by high frequency alternating current (HFAC). Open Microbiol J. 2008;2:32-37.
- Cassanelli C, Roveta S, Cavallini F, Marchese A, Debbia EA, Armanino R. Bactericidal effect of endox against various pathogens. 14th ECCMID; 2004; Prague, Czech Rep. Abstr. P480.
- Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35(3):221-228.
- Figdor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immunol. 2003;18(4):234-239.
- Hubbezoglu I, Zan R, Tunc T, Sumer Z. Antibacterial Efficacy of Aqueous Ozone in Root Canals Infected by Enterococcus faecalis. Jundishapur J Microbiol. 2014;7(7):e11411.
- Kaptan F, Güven EP, Topcuoglu N, Yazici M, Külekçi G. In vitro assessment of the recurrent doses of topical gaseous ozone in the removal of Enterococcus faecalis biofilms in root canals. Niger J Clin Pract. 2014;17(5):573-578.
- Karale R, Thakore A, Shetty V. An evaluation of antibacterial efficacy of 3% sodium hypochlorite, high-frequency alternating current and 2% chlorhexidine on Enterococcus faecalis: An in vitro study. J Conserv Dent. 2011;14(1):2-5.
- Kustarci A, Sümer Z, Altunbaş D, Koşum S. Bactericidal effect of KTP laser irradiation against Enterococcus faecalis compared with gaseous ozone: an ex vivo study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):e73-e79.
- Lendini M, Alemanno E, Migliaretti G, Berutti E. The effect of high-frequency electrical pulses on organic tissue in root canals. Int Endod J. 2005;38(8):531-538.
- Lima SM, Sousa MG, Freire MS, Almeida, et al. Immune Response Profile against Persistent Endodontic Pathogens Candida albicans and Enterococcus faecalis In Vitro. J Endod. 2015;41(7):1061-1065.
- Mehrvarzfar P, Saghiri MA, Asatourian A, et al. Additive effect of a diode laser on the antibacterial activity of 2.5% NaOCl, 2% CHX and MTAD against Enterococcus faecalis contaminating root canals: an in vitro study. J Oral Sci. 2011;53(3):355-360.
- Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M, Nishihara T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol. 2004;19(4):240-246.
- Polydorou O, Pelz K, Hahn P. Antibacterial effect of an ozone device and its comparison with two dentin-bonding systems. Eur J Oral Sci. 2006;114(4):349-353.
- Rôças IN, Siqueira JF, Santos KRN. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30(5):315-320.
- Sedgley CM, Lee EH, Martin MJ, Flannagan SE. Antibiotic resistance gene transfer between Streptococcus gordonii and Enterococcus faecalis in root canals of teeth ex vivo. J Endod. 2008;34(5):570-574.
- Siqueira JF Jr, Rôças IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26(6):331-334.
- Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32(2):93-98.
- Vianna ME, Zilio DM, Ferraz CC, Zaia AA, de Souza-Filho FJ, Gomes BP. Concentration of hydrogen ions in several calcium hydroxide pastes over different periods of time. Braz Dent J. 2009;20(5):382-388.
- Virtej A, MacKenzie CR, Raab WH, Pfeffer K, Barthel CR. Determination of the performance of various root canal disinfection methods after in situ carriage. J Endod. 2007;33(8):926-929.
- Zan R, Hubbezoglu I, Sümer Z, Tunç T, Tanalp J. Antibacterial effects of two different types of laser and aqueous ozone against Enterococcus faecalis in root canals. Photomed Laser Surg. 2013;31(4):150-154.
- Zhang C, Du J, Peng Z. Correlation between Enterococcus faecalis and persistent intraradicular infection compared with primary intraradicular infection: a systematic review. J Endod. 2015;41(8):1207-1213.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Discussion
Discussion
