Dr. Allen Ali Nasseh explains how Triton all-in-one irrigating solution has simplified his procedural setup and increased clinical efficiency.

Dr. Allen Ali Nasseh discusses how to simplify the irrigation process
Experienced endodontists have asserted that the cleaning component of the endodontic triad of cleaning, shaping, and obturation is the most important determinant of clinical success in root canal therapy. However, we tend to spend more time and effort evaluating the latest instrumentation method or finding ways to enhance the final look of our radiographs by incorporating more radiopaque cements into our clinical armamentarium instead of focusing on improving our irrigation protocol and, by proxy, our outcomes.
To make things worse, when we finally decide to focus on irrigation, we find that we are either facing a complicated irrigation protocol shared by many experts on the field or are told that we need expensive, laser-based or sound-based devices that promise to facilitate the irrigation process only if we would be willing to spend large sums, forcing us to either increase our fees or face a greater overhead. So, how can we simplify our irrigation protocol without expensive gadgets and complicated initial and final irrigation protocols?
Before we do set out to simplify our irrigation protocol, it would be worthwhile to review the important concepts that apply to effective irrigation, what chemicals are commonly used, and the objective for their use. Once we have a better grasp of these concepts, we can decide if a simpler solution that addresses our needs is clinically available without exorbitant costs. I’ll briefly discuss some of the physical and chemical parameters in irrigation process and irrigation solutions and potential interactions between the reagents commonly used.
Physical parameters and limitations
The root canal anatomy can be intricate with many curves, fins, and anastomoses. And while the coronal pulp chamber and the coronal root have many dentinal tubules that can act as potential sites of microbial penetration, these spaces constitute a very small volume. The root canal has a volume of 20-40 ml1 with an average of about 0.025 cc. To give you a sense of scale, this volume is equal to the volume of about half a drop of water. Therefore, each cubic centimeter (cc or ml) of irrigation solution will displace 20 to 40 root canal volumes. This is important considering the fact that volume is an important component of effective irrigation. However, we don’t know exactly how much volume replacement is enough for effectively cleaning and whether the chemistry of chemicals used can catalyze this event. We do know that heat increases the kinetic energy of the solution and will therefore catalyze the rate of reaction.2,3
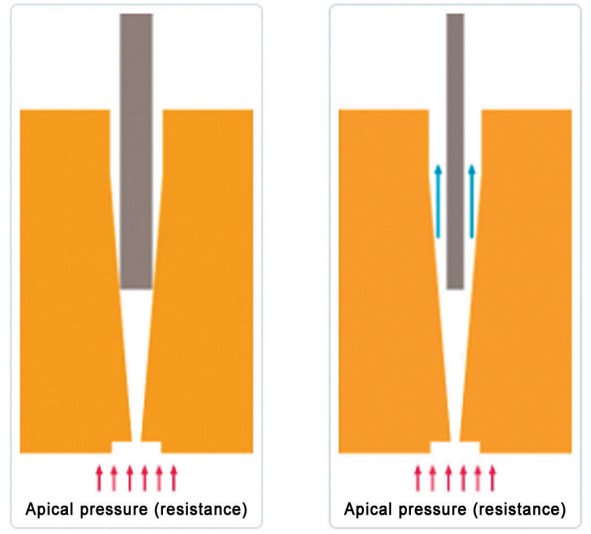
Beyond the volume, the delivery of the solutions into the root canal is also important. Positive pressure irrigation is when the solution is pushed through the syringe with the use of a needle deep in the root canal. Needles with different tip designs distribute the irrigants differently. Currently, close-ended, side-vented needles are the safest needles for use as they reduce the risk of irrigation extrusion from inside the canal. However, while these needles have to be taken deep in the canal in order to be effective, it’s important to use the thinnest needle available and to make sure it’s not binding in the canal during irrigation. In order to help reduce the odds of accidental extrusion, negative pressure systems were recently developed where the vacuum force is moved at the apex with the aid of a thin needle/cannula, and the irrigation solution is deposited coronally in the access opening. This method helps reduce the odds of solution extrusion dramatically. The downside of negative pressure, however, is the ergonomics of the system and the chance of the small suction needle getting blocked prematurely.
Chemical parameters and limitations
To simplify the process of root canal irrigation to its bare essentials, most irrigation solutions are solutions based on either acids, bases, disinfectants, and/or lubricants. The main goal of irrigation is the removal of the macro debris generated during instrumentation and use the aforementioned chemicals in order to achieve the three main objectives of irrigation:
- Dissolve the organic tissue from inside the root canal (pulp, organic portion of dentinal chips, collagen, smear layer, etc.).
- Gently dissolve the inorganic tissues inside the root canal space (spear layer, dentinal chips, and calcifications).
- Disinfect surfaces left behind by destroying all forms of established biofilms inside the root canal.
To date, this has been achieved with the help of a concentration of sodium hypochlorite solution that ranges from 1% to 6% NaOCI (lower concentrations for disinfection only and higher concentrations for additional tissue dissolution). In addition, we’ve used a 17% EDTA solution (17%) to remove the loose inorganic components inside the root canal. Additional lubricants like RC-Prep® (Premier®, Plymouth Meeting, Massachusetts) and also surfactants to reduce surface tension and aid in solution penetration inside dentinal tubules have also been used by some for additional benefits but have not been considered essential to the irrigation process.
One challenge, however, is the fact that NaOCI and EDTA cannot be mixed together as they neutralize and hydrolyze each other within a few minutes after mixing.4 This is why operators have to use two separate syringes for each solution, and if chlorhexidine (CHX) is used in addition, then an additional water rinse is required in between NaOCI and CHX to avoid a toxic precipitate.
Generally, the sodium hypochlorite solution and EDTA solutions are used intermittently throughout the chem-mechanical process by using them back and forth with additional protocols of EDTA at the end to remove the smear layer. This separation has been due to an additional chemical limitation when using NaOCI in teeth. We know that sodium hypochlorite is not only buffered by EDTA, but also buffered and neutralized very quickly upon contact with dentin and dentinal chips. Therefore, the use of EDTA interchangeably throughout the process has been theorized to help dissolve the dentinal chips and therefore help reduce the rate of NaOCI deactivation. But since they cannot be mixed together, they are used in different syringes interchangeably. Lubricants enter the scene as well as per operator’s discretion.
As you can see, these chemical interactions and buffering limitations have created a long and labor-intensive process of using multiple syringes with multiple needles throughout the process with complicated order of operations at the end of the procedure with the main goal of dissolving the dentinal chips with EDTA followed by dissolution of the organic tissue and disinfection by NaOCI. So, are we forced to work through this multiple syringe system and accept this complexity?
A better solution?
While the use of these three basic solutions in alternate syringes has become second nature to most of us, over the past decade chemists from a couple of companies have been working to develop a series of chelating chemicals that can withstand the harsh and corrosive reaction between NaOCI with EDTA by focusing on replacing the EDTA component of irrigation with a series of substitute chelating agents that are less neutralizing to NaOCI. It’s important to note that this scientific effort was made to replace EDTA rather than NaOCI in this process, since NaOCI is considered the gold standard irrigant in the endodontic therapy, primarily due to its simultaneous action on organic tissue dissolution while being an effective disinfectant. As a result, instead of reinventing the wheel by developing a new disinfectant and a new organic solvent, which would have required prospective long-term studies to validate their efficacy, NaOCI was used as the base for a new solution that contains a mix of 11 different gentle chelators, which exhibit resistance to NaOCI and do not buffer NaOCI as quickly as EDTA.

Furthermore, a number of saponification agents and lubricants were also added to the mix so that the final cocktail of solutions can address all three requirements of irrigation and potentially more all in one solution. The resulting irrigation solution is Triton™ (Brasseler USA, Savannah, Georgia). The delivery of the irrigants in a Triton bottle is possible through a unique bottle with a dual barrel delivery system, where 8% NaOCI is mixed 1:1 with a solution of chelating agents, lubricants, surfactants, and detergents. As a result, the final solution drawn into the syringe is a 4% solution of NaOCI with all the additional ingredients in it. Triton solution is stable for 3 to 5 hours after mixing/drawing from the bottle beyond which the NaOCI concentration is considered too low for its intended use. Therefore, Triton is drawn/mixed to use per patient and is stable for 3 to 5 hours after being drawn from the bottle.
The operator can make a decision about how much solution is needed for each case and proceed to draw for use. Each bottle yields a total of 480 ml of solution, which at 6 ml/cc per case (average use by any given clinician), would allow for about 80 cases. The unmixed solution in the bottle has a shelf life of 1 year on the bench top and 2 years in the refrigerator. In my practice, I have found that Triton is actually more cost-effective versus my old protocol using multiple solutions, syringes, and needles. Aside from the material costs, Triton has simplified my procedural setup and increased my clinical efficiency saving me valuable chair time.
A number of independent scientific studies have been performed by various universities around the world, and the studies are on the publication path at the time of writing this article. The results of these studies show excellent disinfection qualities as expected from a NaOCI based solutions. Further synergistic effect is possible as simultaneous application of chelation during disinfection can potentially have a catalytic effect on both processes. This and other results have to be seen. Being a hypochlorite solution, Triton should be kept inside the tooth, and the same care with NaOCI irrigation should be applied here.
While the future of this product is bright, and it may be shown to improve the irrigation/disinfection process, one thing is certain — that the move from multiple syringes and complicated irrigation protocol to a much simpler irrigation protocol, where a single syringe is used from the beginning to the end of the procedure without any sequencing needs, can allow a much more efficient irrigation protocol for most clinicians.
Furthermore, by maintaining 4% sodium hypochlorite as the main active ingredient in Triton, we can apply through precedent the existing body of literature and long-term clinical experience about the efficacy of this solution for disinfection. All the additional benefits and its potential synergy will be a bonus to the clinician.
Check out the details on Triton all-in-one irrigating solution here: https://endopracticeus.com/triton-all-in-one-irrigation-solution-by-brasseler-usa/
- Cardoso FGDR, Martinho FC, Ferreira NS, et al. Correlation Between Volume of Root Canal, Cultivable Bacteria, Bacterial Complexes and Endotoxins in Primary Infection. Braz Dent J. 2019;30(2):117-122.
- Basrani B, Haapasalo M. Update on endodontic irrigating solutions. Endod Topics. 2012;27:74-102.
- Haapasalo M, Shen Y, Wang, Z, Gao Y. Irrigation in endodontics. Br Dent J. 2014;16(6): 299-303.
- Zehnder M. Root canal irrigants. J Endod. 2016;32(5):389-398.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Allen Ali Nasseh, DDS, MMSc, received his dental degree from Northwestern University Dental School in Chicago, Illinois, in 1994 and completed his postdoctoral endodontic training at Harvard School of Dental Medicine in 1997, where he also received a Masters in Medical Sciences (MMSc) degree in the area of bone physiology. He has been a clinical instructor and lecturer in the postdoctoral endodontic program at Harvard School of Dental Medicine since 1994 and the Alumni Editor of Harvard Dental Bulletin. Dr. Nasseh is the endodontic advisor to several educational groups and study clubs and is endodontic editor to several peer-reviewed journals and periodicals. He has published numerous articles and lectures extensively nationally and internationally in surgical and nonsurgical endodontic topics. Dr. Nasseh is in solo private practice (MSEndo.com) in downtown Boston, Massachusetts.
Allen Ali Nasseh, DDS, MMSc, received his dental degree from Northwestern University Dental School in Chicago, Illinois, in 1994 and completed his postdoctoral endodontic training at Harvard School of Dental Medicine in 1997, where he also received a Masters in Medical Sciences (MMSc) degree in the area of bone physiology. He has been a clinical instructor and lecturer in the postdoctoral endodontic program at Harvard School of Dental Medicine since 1994 and the Alumni Editor of Harvard Dental Bulletin. Dr. Nasseh is the endodontic advisor to several educational groups and study clubs and is endodontic editor to several peer-reviewed journals and periodicals. He has published numerous articles and lectures extensively nationally and internationally in surgical and nonsurgical endodontic topics. Dr. Nasseh is in solo private practice (MSEndo.com) in downtown Boston, Massachusetts.