CE Expiration Date: December 21, 2026
CEU (Continuing Education Unit):2 Credit(s)
AGD Code: 157
Educational aims and objectives
This self-instructional course for dentists aims to discuss how to manage pre- and postoperative oral surgery/endodontic procedural pain in patients during the opioid epidemic.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz online at endopracticeus.com to earn 2 hours of CE from reading this article. Correctly answering the questions will demonstrate the reader can:
- Realize the extent of the opioid crisis in the United States.
- Anticipate which cases will require analgesic intervention and to what extent.
- Identify the best analgesia choices in individual patients dependent on patient history and procedure performed.
- Realize a variety of systemic, local, and topical prescriptions, chairside and OTC (over-the-counter) analgesia options for the patient and the practitioner.
Dr. Diana Bronstein shows how to avoid opioid dependence and maintain pain management pre- and postoperatively.
Drs. Diana Bronstein and Rita Steiner discuss protocols to reduce patient dependence on opioids for pain management
Introduction and background
The opioid crisis is a well-documented and reported current event which deserves attention and consideration when practicing daily patient care. With over 9.5 million Americans abusing prescription opioids in 2020 and over 2.7 million with an opioid use disorder, the U.S. Department of Health and Human Services has declared the misuse of opioids a public health emergency.2
Today’s clinicians are aware that the amount of peri- and postoperative opioid use for pain management and their intake duration following surgery are positively associated with chronic opioid use and addiction subsequently. It is one of the top contributors to this epidemic.3-5 The challenge is to reduce opioid use while maintaining adequate pain control.
This article will examine surgical procedures known to increase patients’ risk of developing chronic opioid use and propose protocols for better patient outcomes including reduction of dosage and duration of surgical procedure-related opioid use. Dose increases in both the postoperative inpatient and outpatient settings independently increase the risk of prolonged opioid use,3,6 including opioid naive patients.
Pain management modalities for surgical patients
Managing postoperative pain is an important part of the surgery that involves carefully weighing the risks and benefits since initial and progressive pain control plays a large role in a patient’s overall satisfaction with treatment. A patient experiencing too much pain leads to poor clinical outcomes, while providing access to more than the minimal necessary amount of opioids can initiate chronic dependency.7,8 The challenge is to judge the minimal effective dosage of opioids for adequate pain control successfully while it varies from patient to patient, making it difficult to assess their pain sensitivity objectively.9
“One potential solution to this problem is the use of peripheral nerve blocks. Their use as a replacement for at least some percentage of opioid pain control during and after medical procedures has the potential to reduce opioid use, misuse, and dependency.” Current research suggests that using peripheral nerve block may present viable analgesia.1 According to Cardwell, et al., 2022, the use of pre-surgical peripheral nerve blocks significantly decreases opioid need not only after the procedure, but also in all facets of the surgical process. The most significant reduction in opioid consumption is seen in the first 1-3 days postop, and the patient who received peripheral nerve blocks in the study reported lower pain scores than the control group individuals. It is important to note that this reduction in opioid consumption did not negatively impact patient experience or increase their pain score levels. In fact, it has been shown that the “patients have significantly lower pain scores, higher overall satisfaction, and even prefer the use of blocks when compared to general anesthesia and opioids alone. However, utilization of peripheral nerve blocks is not ubiquitous” while this study produced evidence that peripheral nerve blocks are an effective tool for managing postoperative pain.

Another painful oral surgery sequela is post-extraction dry socket occurrence most often experienced by smokers and non-compliant patients. Dry socket is one of the most common postoperative complications after mandibular tooth extraction, characterized by severe pain and exposed bone. The usual palliative is irrigation of the socket to debride any food or foreign material and packing of the socket with medicated gel or paste to provide pain relief and allow normal wound healing.10
Studies have reported that dry socket pain starts 1–3 days after tooth extraction.17,18 The time it takes for the dry socket to heal varies depending on its severity, but usually, it ranges from 5 to 10 days.19
The management of dry socket has been less controversial18 than its etiology and prevention. Many authors agree that the primary objective is pain control until normal healing occurs as suggested by Fazakerley.16 Systemic analgesics or antibiotics may be necessary or indicated.20 The use of intra-alveolar dressing materials is also suggested in the literature as local palliative treatment,21,22 although it is generally acknowledged that dressings delay the healing of the extraction socket.23

Another treatment modality of dry socket appears to be Platelet-Rich Fibrin (PRF). PRF is characterized by the slow polymerization during its preparation in the centrifuge that generates a fibrin network very similar to the natural one that enhances cell migration and proliferation.13 Choukroun, et al.,15 in France advocated the use of PRF, which is a second-generation platelet concentrate. PRF is a stringently autologous fibrin matrix. Dohan, et al.,14 suggested that PRF addition can correct destructive reactions in the natural process of healing of wound tissues, suggesting that PRF contributes to the immune regulatory mechanism. Choukroun, et al.,15 demonstrated a clinical example in which they used the PRF as a filling material in the extraction socket. There was a significant decrease in pain and the number of socket wall exposure by the third postoperative day; the pain had completely resolved and socket fully epithelialized by the tenth postoperative day. The use of PRF yielded promising results in terms of both pain reduction and improved wound healing which was comparable to the conventional Alveogyl (Septodont) dressing. It may be concluded that PRF is an effective modality for the management of dry socket.10
The studies confirmed that neovascularization and epithelial coverage of the extraction socket can be achieved with the use of PRF. PRF is a reservoir of platelets, leukocytes, cytokines, and growth factors. It is reported to allow the slow release of cytokines, transforming growth factor, platelet-derived growth factor, vascular endothelial growth factor, and epidermal growth factor, which play a vital role for angiogenesis, tissue healing, and cicatrization.14,15
There are further modalities of postoperative morbidity control. Many innovations have made their way into mainstream standard of care. One of the main challenges after extraction, especially postsurgical extractions of impacted 3rd molars, is pain control, and one of the treatments for pain control is low-level laser therapy (LLLT). The study by Santos, et al., 2020, aimed to assess the effectiveness of LLLT for pain control after extraction of lower third molars11 and concluded that LLLT within the parameters determined was effective in reducing the intensity of postoperative pain in third molar surgery, presenting the best results 48 and 72 hours after the procedure.

The application of LLLT can offer greater postoperative comfort and wellbeing to patients, functioning as both an inhibitor of the inflammatory process and a modulator. The working mechanism is by interference of the laser in biochemical and molecular levels, promoting the improvement of clinical signs and symptoms, considering that it stimulates endorphin release, inhibits nociceptive signals, and reduces pain proception. In addition, LLLT may reduce edema and hyperemia, accelerate the wound-healing process, and stimulate bone repair.51,52
With new modalities of pain management, there are also improved conventional pain medication protocols to provide today’s practitioners with evidence-based prescription framework. Since the efficacy and rapid onset of postsurgical oral pain relief are critical to improve clinical outcomes and reduce the risk of excessive dosing with analgesic drugs, another study by Cristalli, et al., 2021, compared analgesic effects of preoperative administration of paracetamol 500 mg plus codeine 30 mg in single-tablet and effervescent formulation to ibuprofen 400 mg, and placebo in the management of moderate-to-severe postoperative pain after mandibular third molar surgery.12
Within the limits of that study, over postoperative 3 days, a statistically significant intensity pain reduction and decreased rescue therapy consumption were recorded in the paracetamol-codeine group than to ibuprofen group. Nevertheless, lower pain intensity at 2 hours post-dose and longer time using rescue therapy was found in the ibuprofen group without statistical significance and without adverse events over the studied period.12,53
A critical property of antiseptic solutions is pain management, especially in terms of trying to limit opioid use. The U.S. opioid public health crisis due to over-prescribing, has subsequently created a drug overuse problem mostly affecting teenagers, leading to a dramatic increase in fatal overdoses.25
It has been more than 5 years since the Food and Drug Administration (FDA), National Institute of Drug Abuse (NIDA), National Institutes of Health (NIH), Drug Enforcement Agency (DEA), the Centers for Disease Control (CDC), and medical and dental organizations such as the American Medical Association (AMA), American Dental Association (ADA),26 and the American Association of Oral and Maxillofacial Surgeons (AAOMS) collectively declared a drastic need to combat their misuse.26,27,28,29,30
Since the mid-1990s, deaths from opioid overdose has more than quadrupled, which parallels the increase in opioid prescriptions written in dental and medical practices,31,32 which is why some countries have entirely banned opioid use in dental practice. In these areas, pain control appears to be manageable. Given that the surgical area is known prior to the procedure, pre-surgical analgesia can be implemented to help reduce the amount of opioid prescriptions in a private practice setting.33,34

Pain perception is initiated in the peripheral nervous system (PNS) before the central nervous systems (CNS) become involved, starting with nociceptors which transmit signals along small myelinated A and unmyelinated C fibers before synapsing in the dorsal horn of the spinal cord.35,37 Signals are then relayed through the thalamus and cortex via the spinothalamic tract of the spinal cord.36
Activity in the dorsal horn can be modulated by psychological factors.35 Active ingredients in StellaLife such as Aconitum, Gelsemium, and Ignatia have shown anxiolytic properties.40,42,43 Anxiolysis is critically important in dental pain management, since patients with anxiety or depression experience more pain from surgery.48 Postoperative pain occurs in two phases: an initial phase with acute pain at the point of noxious stimuli (or incision), and a second phase of prolonged, dull pain around the surgical area.35 The pain stimulus is initiated by inflammatory mediators released at the site of surgery.41,35 The objective of pre- and postoperative analgesia is to decrease inflammatory mediators post-surgery.35,45 Unlike conventional pain management regiments, minimizing postoperative pain with StellaLife® starts 3 days before the procedure. VEGA® Oral Care Recovery Kit by StellaLife has 16 active homeopathic ingredients including Arnica, chamomile, and Aconitum. In a study evaluating the mechanisms of Arnica montana flower methanol extract (AMME) in an arthritic rat model,47 the authors proved that AMME significantly reduced the amount of oxygen free-radicals and pro-inflammatory cytokines such as TNF-Į, IL-1, and IL-6, without the host exhibiting toxic side effects. Interestingly, when compared to the commonly utilized corticosteroid dexamethasone, AMME showed greater therapeutic efficacy in the study.50 Another ingredient in StellaLife is chamomile, which is commonly utilized for pain management due to its anti-inflammatory and anti-nociceptive properties.44,46 The mechanism of action is associated with its ability to inhibit pro-inflammatory cytokines such as TNF-Į, IL-1, IL-6 and IL-8,44 and by its COX inhibition, which is a main mediator of nociception and inflammation.46 Chamomile (Matricaria recutita), which has been used historically as a topical anesthetic, may also function as a selective cycloxegenase (COX)-2 inhibitor.46 It may have a synergistic effect when employed in combination with other non-steroidal anti-inflammatory drugs (NSAIDs), such as diclofenac.46 Chamomile has also been shown to reduce dose-dependent sodium channels, thus decreasing peripheral nerve excitability.39 The anxiolytic and anti-nociceptive mechanism of Aconitum works via blocking voltage-dependent sodium channels.49 It was used in ancient Chinese and Japanese medicine as an analgesic.38 In summary, many of the active ingredients in StellaLife exhibit known and proven anti-inflammatory and anti-nociceptive properties that aid in pain management. Pathways involved include the reduction of pro-inflammatory cytokines, COX inhibition, anxiolytics, and the blockade of neuronal sodium currents. While many of our patients report a soothing effect when using the StellaLife rinse and gel, more research is necessary to evaluate how these ingredients work synergistically to control pain by pre- and postoperative applications.
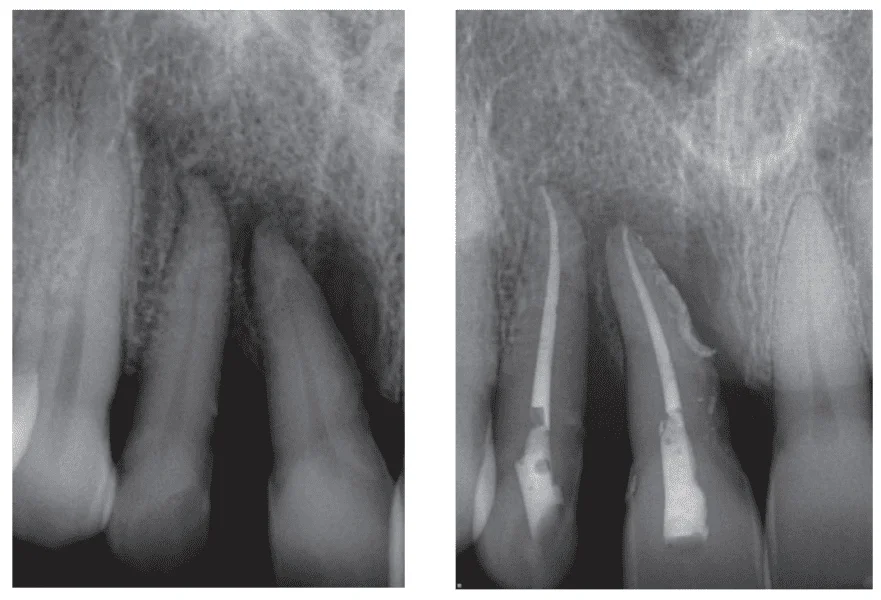
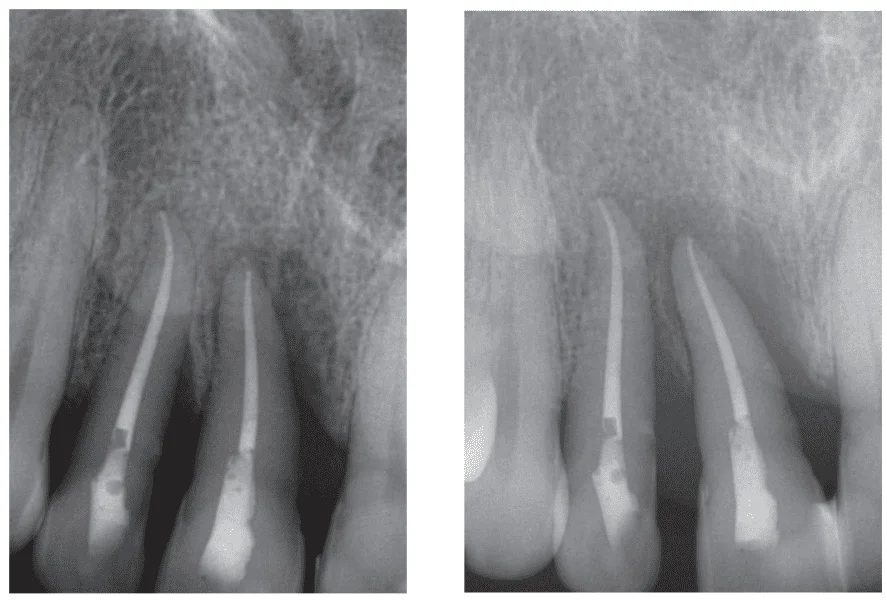
Figures 3-10 illustrate the perio-endo case of a 51-year-old male MMA fighter. Following trauma to his teeth Nos. 7 and 8 which was treated conservatively with root canal therapy and scaling and root planing, surgical management with incision, drainage and curettage were also performed, and consequently, postoperative pain management needed to be applied.
Dr. Fernando J. Meza writes about pain management with the use of lasers during root canal procedures. Read his article here: https://endopracticeus.com/using-dental-lasers-to-decrease-pain-and-fear-for-patients/
References
- Cardwell TW, Zabala V, Mineo J, Ochner CN. The Effects of Perioperative Peripheral Nerve Blocks on Peri- and Postoperative Opioid Use and Pain Management. Am Surg. 2022;88(12):2842–2850.
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 national survey on drug use and health. Published October 2021. https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf. Accessed January 6, 2023.
- Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, Bohnert ASB, Kheterpal S, Nallamothu BK. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017 Jun 21;152(6):e170504.
- Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. 2014 Feb 11;348:g1251.
- Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
- Ruddell JH, Reid DBC, Shah KN, Shapiro BH, Akelman E, Cohen EM, Daniels AH. Larger Initial Opioid Prescriptions Following Total Joint Arthroplasty Are Associated with Greater Risk of Prolonged Use. J Bone Joint Surg Am. 2021 Jan 20;103(2):106-114.
- Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287-2298.
- Demsey D, Carr NJ, Clarke H, Vipler S. Managing opioid addiction risk in plastic surgery during the perioperative period. Plast Reconstr Surg. 2017;140(4):613e-619e.
- Younger J, McCue R, Mackey S. Pain outcomes: A brief review of instruments and techniques. Curr Pain Headache Rep. 2009;13(1):39-43.
- Keshini MP, Shetty SK, Sundar S, Chandan SN, Manjula S. Assessment of Healing Using Alvogyl and Platelet Rich Fibrin in Patients with Dry Socket – An Evaluative Study. Ann Maxillofac Surg. 2020 Jul-Dec;10(2):320-324.
- Santos PL, Marotto, AP, Zatta da Silva T, Bottura MP, Valencise M, Marques DO, Queiroz TP. Is Low-Level Laser Therapy Effective for Pain Control After the Surgical Removal of Unerupted Third Molars? A Randomized Trial. J Oral Maxillofac Surg. 2020 Feb;78(2): 184–189.
- La Monaca G, Pranno N, Annibali S, Polimeni A, Pompa G, Vozza I, Cristalli MP. Comparative Analgesic Effects Of Single-Dose Preoperative Administration Of Paracetamol (Acetaminophen) 500 Mg Plus Codeine 30 Mg And Ibuprofen 400 Mg On Pain After Third Molar Surgery. J Evid Based Dent Pract. 2021 Dec;21(4):101611.
- Al-Hamed FS, Tawfik MA, Abdelfadil E. Clinical effects of platelet rich fibrin (PRF) following surgical extraction of lower third molar. Saudi J Dent Res. 2017;8(1,2):19-25.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e45-50.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):e56-60.
- Fazakerley M, Field EA. Dry socket: a painful post-extraction complication (a review). Dent Update. 1991 Jan-Feb;18(1):31-34.
- Fridrich KL, Olson RA. Alveolar osteitis following surgical removal of mandibular third molars. Anesth Prog. 1990 Jan-Feb;37(1):32-41.
- Nitzan DW. On the genesis of “dry socket”. J Oral Maxillofac Surg. 1983 Nov;41(11): 706-710.
- Blum IR. Contemporary views on dry socket (alveolar osteitis): a clinical appraisal of standardization, aetiopathogenesis and management: a critical review. Int J Oral Maxillofac Surg. 2002 Jun;31(3):309-317.
- Heasman PA, Jacobs DJ. A clinical investigation into the incidence of dry socket. Br J Oral Maxillofac Surg. 1984 Apr;22(2):115-122.
- Vezeau PJ. Dental extraction wound management: medicating postextraction sockets. J Oral Maxillofac Surg. 2000 May;58(5):531-537.
- Swanson AE. A double-blind study on the effectiveness of tetracycline in reducing the incidence of fibrinolytic alveolitis. J Oral Maxillofac Surg. 1989 Feb;47(2):165-167.
- Bloomer CR. Alveolar osteitis prevention by immediate placement of medicated packing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Sep;90(3):282-284.
- Estrin NE, Romanos GE, Tatch W, Pikos M, Miron RJ. Biological Characterization, Properties, and Clinical Use of a Novel Homeopathic Antiseptic Oral Recovery Kit: A Narrative Review. Oral Health Prev Dent. 2022 Nov 30;20(1):485-499.
- Rutkow L, Vernick JS. Emergency Legal Authority and the Opioid Crisis. N Engl J Med. 2017 Dec 28;377(26):2512-2514.
- American Dental Association (ADA). American Dental Association announces new policy to combat opioid epidemic. 2018. https://www.prnewswire.com/news-releases/american-dental-association-announces-new-policy-to-combat-opioid-epidemic-300618928.html Accessed January 9, 2023.
- Califf RM, Woodcock J, Ostroff S. A Proactive Response to Prescription Opioid Abuse. N Engl J Med. 2016 Apr 14;374(15):1480-1485.
- Centers for Disease Control and Prevention. Opioid overdose: Understanding the epidemic. Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017. Reviewed June 1, 2022.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016. 2016 Apr 19;315(15):1624-1645.
- Hupp JR. Opioids: Combating Misuse While Properly Caring for Our Patients. J Oral Maxillofac Surg. 2019 Apr;77(4):669-670.
- National Institute on Drug Abuse. Overdose death rates. National Institute on Drug Abuse, National Institutes of Health, 2017. Updated January 20, 2022.
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths–United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016 Jan 1;64(50-51):1378-1382.
- Lee CYS, Suzuki JB. The efficacy of preemptive analgesia using a non-opioid alternative therapy regimen on postoperative analgesia following block bone graft surgery of the mandible: A prospective pilot study in pain management in response to the opioid epidemic. Clin J Pharmacol Pharmacother. 2019;1(2):1006.
- Tatch W. Opioid Prescribing Can Be Reduced in Oral and Maxillofacial Surgery Practice. J Oral Maxillofac Surg. 2019 Sep;77(9):1771-1775
- Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician. 2001 May 15;63(10):1979-1984.
- Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, Hema PS, Chaturvedi MM, Nair M, Aggarwal BB. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010 Nov 12;285(46):35406-35417.
- Pogatzki-Zahn EM, Zahn PK. From preemptive to preventive analgesia. Curr Opin Anaesthesiol. 2006 Oct;19(5):551-555.
- Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol. 1998 Oct;56(2):211-235.
- Alves Ade M, Gonçalves JC, Cruz JS, Araújo DA. Evaluation of the sesquiterpene (-)-alpha-bisabolol as a novel peripheral nervous blocker. Neurosci Lett. 2010 Mar 12;472(1):11-15.
- Bhat NP, Sairoz DC, Shetty A, Quadros L, Krishnan H, Bakthavatchalam P. Efficacy of Aconite and Ignatia as an anxiolytic-In vivo study. Int J Res Pharmaceut Sci. 2021;12(2):1484–1489.
- Dahl JB, Kehlet H. Preventive analgesia. Curr Opin Anaesthesiol. 2011 Jun;24(3): 331-338.
- Magnani P, Conforti A, Zanolin E, Marzotto M, Bellavite P. Dose-effect study of Gelsemium sempervirens in high dilutions on anxiety-related responses in mice. Psychopharmacology (Berl). 2010 Jul;210(4):533-545.
- Marzotto M, Conforti A, Magnani P, Zanolin ME, Bellavite P. Effects of Ignatia amara in mouse behavioural models. 2012 Jan;101(1):57-67.
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother Res. 2006 Jul;20(7):519-530.
- Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005 Mar;100(3): 757-773.
- Ortiz MI, Fernández-Martínez E, Soria-Jasso LE, Lucas-Gómez I, Villagómez-Ibarra R, González-García MP, Castañeda-Hernández G, Salinas-Caballero M. Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed Pharmacother. 2016 Mar;78:248-256.
- Sharma S, Arif M, Nirala RK, Gupta R, Thakur SC. Cumulative therapeutic effects of phytochemicals in Arnica montana flower extract alleviated collagen-induced arthritis: inhibition of both pro-inflammatory mediators and oxidative stress. J Sci Food Agric. 2016 Mar 30;96(5):1500-1510.
- Taenzer P, Melzack R, Jeans ME. Influence of psychological factors on postoperative pain, mood and analgesic requirements. 1986 Mar;24(3):331-342.
- Wang CF, Gerner P, Wang SY, Wang GK. Bulleyaconitine A isolated from aconitum plant displays long-acting local anesthetic properties in vitro and in vivo. 2007 Jul;107(1):82-90.
- Sharma SM, Anderson M, Schoop SR, Hudson JB. Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce): dual actions against respiratory bacteria. 2010 Jul;17(8-9):563-568.
- Karu T. High-tech helps to estimate cellular mechanisms of low power laser therapy. Lasers Surg Med. 2004;34(4):298-299.
- Medrado AR, Pugliese LS, Reis SR, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med. 2003;32(3):239-244.
- Bronstein D, Suzuki JB. Post Operative Pain Management During the Age of Opioid Crisis. Oral Health. https://www.oralhealthgroup.com/features/post-operative-pain-management-during-the-age-of-opioid-crisis/ Accessed January 9, 2023.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Diana Bronstein, DDS, MS, MS, MS, has been a Clinical Professor, Associate Program Director and Faculty in the Department of Periodontology and at the Advanced Education of General Dentistry Department at Nova Southeastern University, College of Dental Medicine. She is double boarded as Diplomate by the American Board of Periodontology and Implant Dentistry (ABP) in Periodontology and Dental Implant Surgery, and she is a Diplomate and Fellow of the International Congress of Oral Implantologists (ICOI). She co-authored the third and fourth edition of Misch’s and Resnik’s Contemporary Implant Dentistry volumes. Dr. Bronstein has a Diploma in Clinical Homeopathy which she practices upon patient request adjunctively to standard of care during her periodontal and surgical dental practice.
Diana Bronstein, DDS, MS, MS, MS, has been a Clinical Professor, Associate Program Director and Faculty in the Department of Periodontology and at the Advanced Education of General Dentistry Department at Nova Southeastern University, College of Dental Medicine. She is double boarded as Diplomate by the American Board of Periodontology and Implant Dentistry (ABP) in Periodontology and Dental Implant Surgery, and she is a Diplomate and Fellow of the International Congress of Oral Implantologists (ICOI). She co-authored the third and fourth edition of Misch’s and Resnik’s Contemporary Implant Dentistry volumes. Dr. Bronstein has a Diploma in Clinical Homeopathy which she practices upon patient request adjunctively to standard of care during her periodontal and surgical dental practice. Rita Steiner, DMD, has been a dentist since 1994 and an endodontist since 2004. She has been teaching at the VA Medical Center in Miami, Florida and is an adjunct clinical assistant professor at Nova Southeastern University (NSU) College of Dental Medicine (CDM) since 2012. She currently serves as faculty at the Department of Advanced Education in General Dentistry (AEGD). Dr. Steiner was also president of the North Dade -Miami Beach Dental Association 2018-2019.
Rita Steiner, DMD, has been a dentist since 1994 and an endodontist since 2004. She has been teaching at the VA Medical Center in Miami, Florida and is an adjunct clinical assistant professor at Nova Southeastern University (NSU) College of Dental Medicine (CDM) since 2012. She currently serves as faculty at the Department of Advanced Education in General Dentistry (AEGD). Dr. Steiner was also president of the North Dade -Miami Beach Dental Association 2018-2019.