Dr. Jorge Alberdi discusses why MTA was his choice for treatments in these cases
Abstract
Multiple techniques and materials have been described for apexogenesis and apexification in immature permanent teeth. Two cases in which mineral trioxide aggregate (MTA) has been used as the material are described herein.
[userloggedin]
In the first case, vital pulp capping was achieved in the left second mandibular premolar with vital pulp and a deep carious lesion. Dental pulp vitality was conserved, and apexogenesis treatment was successful. The second case had a right maxillary lateral incisor with necrotic pulp and an immature root. An MTA apical plug was the treatment choice. Treatment was successful in both cases, as evidenced on clinical and radiographic assessments and no observed complications during follow-up visits. Clinical aspects of apexogenesis and apexification and considerations about MTA have also been discussed.
Introduction
Dental trauma and caries are the most frequent challenges of the developing tooth. Maintenance of pulp vitality in immature permanent teeth allows for complete physiological root development.1 Accurate diagnosis through clinical and radiographic evaluations will suggest proper treatment for the affected tooth. Radiographic evaluation is essential to determine the maturity of the developing root, and clinical evaluation is essential to determine the history of the pathology and pulp vitality.2
When pulp vitality is maintained, apexogenesis is the suggested treatment to promote natural root development. Depending on the extent of inflammation, pulp capping, shallow pulpotomy, or conventional pulpotomy may be suggested.1,2 Pulp capping was the treatment method in case 1 presented in this report. There are long-term prognostic advantages of this treatment outcome over the apexification procedure. MTA has become the new material of choice for pulp capping.4 These advantages include the fact that tooth structure formed has a greater structural integrity, as the tooth is more resistant to vertical fracture.3,5 If pulpal necrosis occurs in immature teeth, an alternative approach must be used because of the presence of an open apex. Young, pulpless teeth often have thin and fragile walls, which make adequate cleaning and apical sealing difficult and increases the potential for root fracture.2-3,5 Apexification is defined as “a method of inducing a calcified barrier in a root with an open apex or the continued apical development of an incompletely formed root in teeth with necrotic pulp.”2 In these cases, the apical filling material used may induce the formation of a hard tissue barrier, but root length is not improved.6,7 However, this paradigm has been challenged by recent reports showing that immature teeth clinically diagnosed with necrotic pulp can undergo apexogenesis. That means the continuation of root development, which leads to normal root length, apical closure, and a greater structural integrity. In these cases of necrotic pulp, the procedure is described as regenerative endodontic treatment or revascularization.8,9
In 1999, Torabinejad and Chivian described multiple clinical applications of MTA, including capping of pulps in reversible pulpitis and apexification by means of an apical plug in immature roots with a necrotic pulp.10 MTA is a powder that consists of fine hydrophilic particles that harden in the presence of moisture. Hydration of the powder results in a colloidal gel with a pH of 12.5 that solidifies into a hard structure. The setting time for the cement is around 4 hours. MTA has been recognized as a bioactive material that induces and supports tissue mineralization, is a good sealant, and is biocompatible. In addition, MTA is insoluble in tissue fluids, dimensionally stable, and has an adequate radiopacity.10-15 It has been suggested that biocompatibility and sealing ability of MTA originate from the physicochemical reactions between MTA and dentin.11 The study of the physicochemical interaction between MTA and root canal walls revealed that MTA appeared to bond chemically to dentin via a diffusion-controlled reaction between its apatitic surface and dentin, when it is placed against dentin walls.16 Investigation of the bond strength between MTA and dentin will reveal the value of adhesion between them.17
Previously described features make MTA the optimal material for both apexogenesis and apexification. Historically, calcium hydroxide (CaOH) has been the preferred material for apexogenesis and apexification.14 Both therapies require long-term follow-up with clinical and radiographic evaluations to confirm treatment success.
Case report 1: apexogenesis
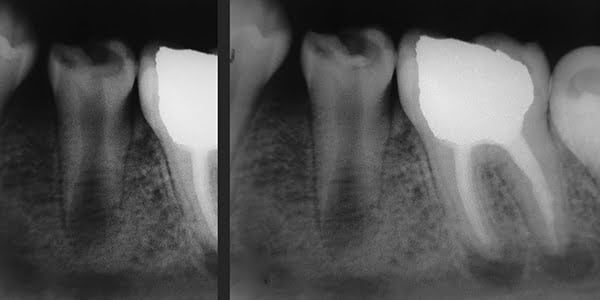
The patient is a 10-year-old boy with a 6-month history of caries and pain in the left second mandibular premolar (tooth No. 20). The patient complained of sensitivity to cold beverages and pain during mastication; he did not report spontaneous pain. Clinical examination showed deep crown caries and minimal pulpal exposure. The tooth was not sensitive on the percussion test. Radiographic examination showed a carious lesion in contact with pulp chamber cavity and a short immature root (Figure 1A). The patient’s medical history was contributory.
Under local anesthesia with 2% lidocaine/1:50,000 epinephrine (Indican, Sidus, Argentina) and rubber dam isolation, caries was removed using a round No. 7 carbide bur (SS White®), with rapid and copious water sprays to prevent heat damage to the subjacent pulp. The cleaned dentin area was irrigated with 2.5% sodium hypochlorite, with saline solution used on last irrigation. Hemostasis was achieved by gentle placement of a sterile cotton pellet moistened with saline solution over the pulp exposure. Following the manufacturer’s instructions, ProRoot® MTA (Dentsply Maillefer, Ballaigues, Switzerland) powder and liquid were mixed to achieve adequate consistency. An approximately 1-mm thick layer of MTA was placed over the exposed pulp exposure using a small resin spatula and was gently compressed with a dry cotton pellet. Then the tooth was permanently filled with Vitremer™ (3M ESPE) (Figure 1B), a glass ionomer.
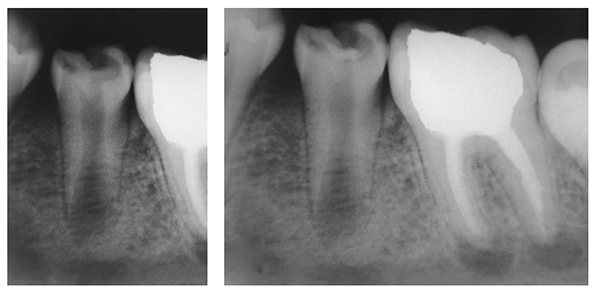
At 1-week follow-up, the tooth was functional and nonsymptomatic, with no evident clinical signs. Natural development of root was observed, and formation of a calcified bridge beneath MTA cement was observed on radiographs obtained at follow-up sessions in 5 months (Figure 2A), 1 year 3 months (Figure 2B), and 1 year 9 months after the procedure (Figure 2C). At a follow-up visit after 2 years 5 months, the root was completely developed, and vital pulp was maintained (Figure 3).

Case report 2: apexification
The patient was a 9-year-old girl with a noncontributory medical history. Two months prior, she had swelling and pain in the right maxillary lateral incisor (tooth No. 7). She was on amoxicillin 500 mg, recently prescribed by her referring dentist. There was no current evidence of swelling or tooth mobility upon presentation. However, a draining sinus tract was seen in the mucolabial fold near the apex. Thermal and electric pulp tests confirmed that tooth No. 7 was nonvital, whereas all adjacent teeth contained vital pulps. The radiograph revealed that tooth No. 7 had an incompletely formed root with an immature apex, surrounded by a periradicular rarefaction (Figure 4A). With her mother’s concurrence, apexification root canal therapy with CaOH was performed. The access cavity was prepared, and the canal was lightly shaped with manual K-files (Dentsply Maillefer, Ballaigues, Switzerland) because of the thickness of the walls. Before shaping, a tentative root canal length was determined using an electronic apical locator and radiographs. It was difficult to determine the precise root canal length because of the opening of the apex. Irrigation was performed using 2.5% sodium hypochlorite (NaOCl) and sterile saline solution. Once chemo-mechanical preparation was finished, the root canal was dried with sterile paper points and dressed with CaOH paste (Farmadental, Argentina). A sterile, dried-cotton pellet was placed into the access cavity and filled using glass ionomer Vitremer (3M ESPE).
The sinus tract was resolved 4 days after the start of treatment, and the patient was instructed to discontinue amoxicillin. Attempts to schedule treatments at 2-month intervals were unsuccessful because of the patient’s parent’s noncooperation. On the 7th month reexamination after the first visit, her mother expressed the inability to continue further treatment because the family had moved to another city, and it was impossible for them to transport the patient to the dental office to continue the treatment. Hence, canal filling with an apical MTA plug and subsequent clinical and radiographic follow-ups were recommended, to which the patient’s mother agreed. After removal of the restorations, a tentative root canal length was determined again using an electronic apical locator and radiographs. The root canal was shaped and cleaned using hand files and 2.5% sodium hypochlorite, and dried with sterile paper points.
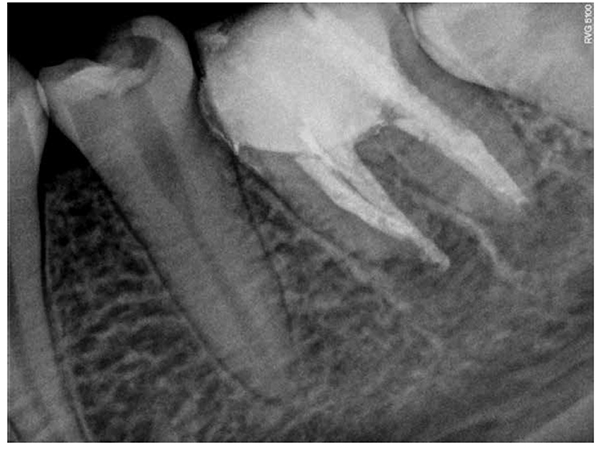
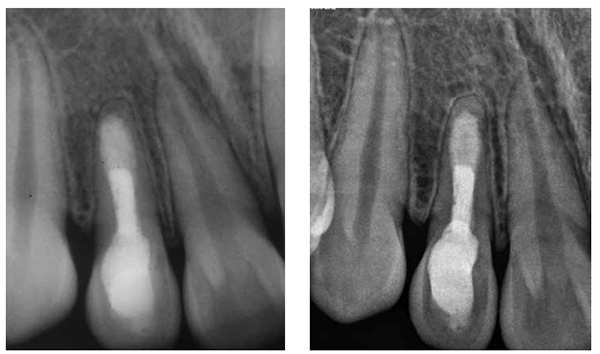
Then a thick mixture of ProRoot MTA (Dentsply Maillefer, Ballaigues, Switzerland) was prepared and applied to the apical portion of the canal using a Messing syringe, calibrated to 2 mm less than the working length, to introduce the MTA in the apical portion. The MTA was condensed vertically with Machtou pluggers (Dentsply Maillefer, Ballaigues, Switzerland) No. 2 and No. 3 and the rear end of sterilized paper points. During this procedure, radiographs were obtained to evaluate the conformation of the MTA plug and its placement in relation to the apical extent of the root structure (Figures 5A-5B). The thickness of the MTA was approximately 3 mm to 4 mm at the apical portion of the root. A moist cotton pellet was placed in the canal, and the access cavity was closed with a temporary restoration material, Cavit™ G (3M ESPE) for 4 hours. The patient returned to the dental office 4 hours later, and Cavit G and the moist cotton pellet were removed. The rest of the canal was filled with flow gutta percha dispensed by Calamus® Dual (Dentsply Maillefer, Ballaigues, Switzerland). The access cavity was sealed with Vitremer (3M ESPE) for final restoration (Figure 6).
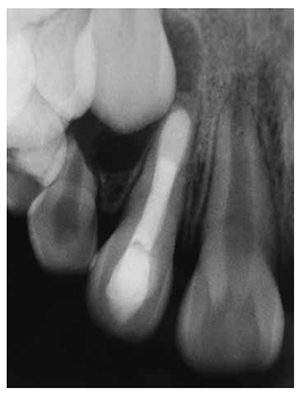
The tooth has been asymptomatic since the final obturation of the root canal. Radiographs obtained at follow-up visits both after 5 years (Figure 7A) and 7 years 5 months (Figure 7B) demonstrated the regeneration of periradicular tissue and revealed a hard tissue apical barrier around the MTA plug.

Discussion
In case 1, apexogenesis with MTA was confirmed at the follow-up visit after 2 years 5 months. No evidence of periapical pathology was noted during the follow-up period.
The main difficulty in treating permanent immature teeth is the ability to predictably diagnose the state of dental pulpal health and, consequently, the ability to predict its healing. The contemporary tests available to the clinician make it difficult to accurately predict the degree of pulpal degeneration before starting treatment. However, the clinician’s skill in assessing pulpal tissue health is of importance. Currently, to control pulpal hemorrhage, NaOCl between 2.5% and 5.5% is the best choice. 1,2-4
Vital pulp capping with MTA in apexo-genesis has superior long-term sealing ability and stimulates formation of a higher quality and greater amount of reparative dentin.2,4,10 The calcified bridge formed by MTA is continuous and has no evidence of tunnel defects, similar to that formed by CaOH. The presence of tunnel defects or the formation of a permeable dentin bridge does not allow a hermetic seal to the underlying pulp. These defects can serve as pathways for bacterial leakage, which deteriorates dental pulp health and can lead to further endodontic treatment necessary due to possible infection or inflammation of the pulpal tissue due to incomplete sealing.5,18 In addition, pulpal inflammation occurs more frequently in dental pulps capped with CaOH compared to those capped with MTA.4,10-12,18 MTA has demonstrated the ability to induce hard tissue formation in pulpal tissues when used as either a direct pulp capping or a pulpotomy material.4,10,19
Histologic evaluation in animal and human studies has revealed that MTA stimulates reparative dentin development, with thick dentinal bridging, negligible inflammation, and minimal hyperemia. MTA also appears to induce the formation of a dentin bridge at a faster rate than CaOH.1,13,21
In case 2, apexogenesis with MTA was confirmed at 5 years after the placement of the MTA apical plug, as the patient declined preceding follow-up visits.
Nonclinical and clinical studies have reported good results with a variety of techniques. The most significant reason for long-term CaOH therapy failure is inadequate time for completion and patient disregard toward follow-up visits. This relates to the time needed for CaOH to work to close the immature apical of the tooth requiring multiple visits over a sometimes long period of time. The average length of time for apical barrier formation with CaOH is approximately between 3 and 24 months.2-14-22-23 Another major disadvantage of the apexification with CaOH is the effect of long-term application of CaOH on the structural integrity of the root dentin. Several studies have demonstrated that with longer exposures of dentin to CaOH, its ability to resist fracture is significantly decreased. In addition to this tissue structure alteration, thin dentin at the cervical region is another causative factor of root fracture at the cervical third of the root. 5,7,11 Particularly regarding apexification, the disadvantages of traditional, long-term, multiple-visit-requiring CaOH therapy include variability in treatment time, unpredictability of formation of apical barrier, difficulty in following up with patients, and delayed treatment.2,11-14
Overall, the results of several studies show that MTA plugs are effective in treating immature permanent teeth with necrotic pulps.19-21 The advantages of apexification with an MTA plug are reduced treatment time — one-visit apexification — and a more predictable barrier formation. The limitation, similar to that of CaOH therapy, is that placement of an apical plug does not account for continued root development along the entire root length.10-14,20,24
Complete root development requires a viable pulp containing cells that can differentiate into dentin-producing odontoblasts. Ongoing studies are aiming at identifying procedures and materials that allow pulp regeneration.3,8,9
Conclusions
Successful treatment as described in this report suggests that MTA may be a better choice for apexification and apexogenesis treatments.
Acknowledgments
The author thanks Prof. Dr. Fernando Goldberg, Buenos Aires, Argentina, and Dra. Arminia Baroffi, Rosario, Argentina, for providing valuable advice during his endodontic career.
[/userloggedin]
[userloggedout][/userloggedout]
- Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives – permanent teeth. J Endod. 2008;34(7 Suppl): S25-S28.
- Shabahang S. Treatment options: apexogenesis and apexification. J Endod. 2013;39(3 Suppl):S26-S29.
- Karapinar-Kazandag M, Basrani B, Tom-Kun Yamagishi V, Azarpazhooh A, Friedman S2. Fracture resistance of simulated immature tooth roots reinforced with MTA or restorative materials. Dent Traumatol. 2015;doi: 10.1111/edt.12230. [Epub ahead of print].
- Boksman L, and Friedman M. MTA: the new material of choice for pulp capping. Endodontic Practice US. 2014;7(2):20-25.
- Nosrat A, Asgary S. Apexogenesis treatment with a new endodontic cement: a case report. J Endod. 2010;36(5):912-914.
- Hayashi M, Shimizu A, Ebisu S. MTA for obturation of mandibular central incisors with open apices: case report. J Endod. 2004;30(2):120-122.
- Sarris, S, Tahmassebi JF, Duggal, MS, Cross IA. A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children-a pilot study. Dent Traumatol. 2008; 24(1):79-85.
- Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011;37(4): 562-567.
- Wang Y, Zhu X, Zhang C. Pulp revascularization on permanent teeth with open apices in a middle-aged patient. J Endod. 2015;41(9):1571-1575.
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25(3):197-205.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16-27.
- Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-part II: leakage and biocompatibility investigations. J Endod. 2010;36(2):190-202.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
- Bakland LK Andreasen JO. Will mineral trioxide aggregate replace calcium hydroxide in treating pulpal and periodontal healing complications subsequent to dental trauma? A review. Dent Traumatol. 2012;28(1):25–32.
- Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35(6):777-790.
- Sarkar NK1, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005; 31(2):97-100.
- Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod. 2010;36(5):871–874.
- Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(4):609–614.
- Moore A, Howley MF, O’Connell AC. Treatment of open apex teeth using two types of white mineral trioxide aggregate after initial dressing with calcium hydroxide in children. Dent Traumatol. 2011;27(3):166–173.
- Selden HS. Apexification: an interesting case. J Endod. 2002; 28(1): 44-45.
- Cho SY, Seo DG, Lee SJ, Lee J, Lee SJ, Jung IY. Prognostic factors for clinical outcomes according to time after direct pulp capping. J Endod. 2013; 39(3): 327-331.
- Sheehy EC, Roberts GJ. Use of calcium hydroxide for apical barrier formation and healing in non-vital immature permanent teeth: a review. Br Dent J. 1997;183(7):241-6.
- Walia T, Chawla HS, Gauba K. Management of wide open apices in non-vital permanent teeth with Ca(OH)2 paste. J Clin Pediatr Dent. 2000;25(1): 51-6.
- Mente J, Hage N, Pfefferle T, Koch MJ, Dreyhaupt J, Staehle HJ, Friedman S. Mineral trioxide aggregate apical plugs in teeth with open apical foramina: a retrospective analysis of treatment outcome. J Endod. 2009;35(10):1354-1358.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..