Dr. James Prichard presents a clinical case in which he examines the treatment of acute symptomatic irreversible pulpitis
 A patient presented with acute severe pain from the upper right quadrant. It was poorly localized, and the patient stated that the pain radiated into the ear and the cheek on the right-hand side. Pain was spontaneous and not responding well to over-the-counter analgesics (Ibuprofen 400mg, qds), and had been gradually getting worse over the last 48 hours. The patient was experiencing sleep disturbance, and the pain came on in waves. There was extreme sensitivity to cold stimulus, but not so painful with hot stimulus.
A patient presented with acute severe pain from the upper right quadrant. It was poorly localized, and the patient stated that the pain radiated into the ear and the cheek on the right-hand side. Pain was spontaneous and not responding well to over-the-counter analgesics (Ibuprofen 400mg, qds), and had been gradually getting worse over the last 48 hours. The patient was experiencing sleep disturbance, and the pain came on in waves. There was extreme sensitivity to cold stimulus, but not so painful with hot stimulus.
[userloggedin]
Examination
 On examination, the upper right first and second molars were restored with amalgam. There was no pocketing or mobility and no tenderness to percussion, nor tenderness in the buccal or palatal sulcus. I performed a sensibility test with Endo-Frost (Roeko) UR7 +ve, UR6 ++ and triggered the patient’s toothache. The upper right first molar had a pinretained restoration, 25% bone loss mesially and distally, no obvious caries, a possible furcal radiolucency but no obvious periapical radiolucency at the root apices. The pulp chamber was reduced in size, and the canals were not obviously visible. The mesial root exhibited severe curvature in excess of 30º (Schneider, 1971) (Figure 1B) towards the distal aspect. The sinus outline appeared to be low and close to the roots. The diagnosis was acute irreversible symptomatic pulpitis from the upper right first molar. The treatment options included root canal treatment or extraction. The patient opted for root canal treatment.
On examination, the upper right first and second molars were restored with amalgam. There was no pocketing or mobility and no tenderness to percussion, nor tenderness in the buccal or palatal sulcus. I performed a sensibility test with Endo-Frost (Roeko) UR7 +ve, UR6 ++ and triggered the patient’s toothache. The upper right first molar had a pinretained restoration, 25% bone loss mesially and distally, no obvious caries, a possible furcal radiolucency but no obvious periapical radiolucency at the root apices. The pulp chamber was reduced in size, and the canals were not obviously visible. The mesial root exhibited severe curvature in excess of 30º (Schneider, 1971) (Figure 1B) towards the distal aspect. The sinus outline appeared to be low and close to the roots. The diagnosis was acute irreversible symptomatic pulpitis from the upper right first molar. The treatment options included root canal treatment or extraction. The patient opted for root canal treatment.
Treatment
Anesthesia was achieved with 1×2.2ml Lignospan® (Septodont) (2% Lidocaine, 1:80,000 adrenaline) via buccal and palatal infiltration and isolation achieved with non-latex dam (3M) and sealed with OraSeal® (Optident) caulking agent. Access was performed with a short tungsten carbide bur and the pulp chamber de-roofed with a safe-ended tapered tungsten carbide bur (Schottlander). There was a pulp stone present in the chamber over the palatal root canal (Figures 2A and 2B), which was removed with canal access preparation (CAP 1) ultrasonic tip (Satelec UK), and three canals were immediately identified with a DG16 endodontic probe. Before canal shaping was performed, the coronal two-thirds was explored with a size 10 K-flex file. Shaping was performed as follows: Scout RaCe (Schottlander) sizes 10/.02, 15/.02, and 20/.02 (Figure 3) were used in an NSK Endo-Mate running at 1,000 rpm to estimated working length using 3% sodium hypochlorite (Schottlander) as the lubricant and irrigant. The irrigant was delivered with a 27G side vented Monoject® needle attached to a 3ml syringe. The canal lengths were determined electronically with an apex locator (MedicNRG) using a size 10 K-Flex File (SybronEndo) and shaped with BioRaCe (Schottlander) BRO, BR1, BR2, BR3, and BR4 sequentially to length irrigating with 3ml 3% NaOCl between each file. After shaping, the root canals were cleaned with the Irrisafe™ Passive Ultrasonic Irrigation tip (Satelec) for three cycles of 20 seconds per canal replenishing the irrigant between each cycle (Figure 4). Following which, a soak was performed with 17% EDTA (Schottlander) for 60 seconds delivered as before, and the final flush was made with 3% NaOCl.
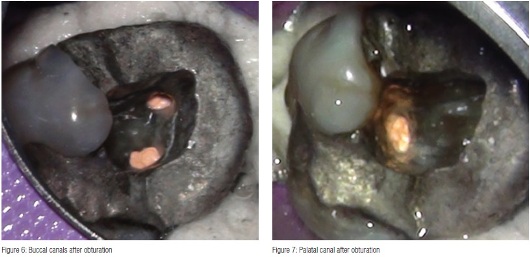
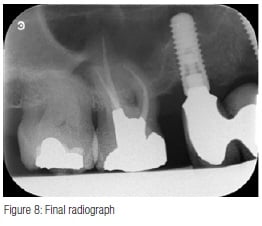 Obturation was performed with TotalFill® BC Sealer™ (Schottlander) and size 35/.04 TotalFill gutta percha cones impregnated with bioceramic. The cones were sized to fit each individual canal with good tug back in canals still wet with 3% NaOCI. The canals were dried with 35/.04 paper points (Schottlander), the cones coated with Total- Fill BC Sealer (Figure 5) and seated into the canals, withdrawn halfway and reseated. The coronal portion of the cones were then removed with a heated instrument and packed gently into the canal orifices (Figures 6 and 7), and the access cavity cleaned by washing with a three-in-one triple syringe. A Nayyar amalgam core was placed, the dam removed, and the occlusion checked. A final radiograph was taken (Figure 8) showing a well-condensed root canal filling in all three canals extending to length with a welladapted coronal restoration.
Obturation was performed with TotalFill® BC Sealer™ (Schottlander) and size 35/.04 TotalFill gutta percha cones impregnated with bioceramic. The cones were sized to fit each individual canal with good tug back in canals still wet with 3% NaOCI. The canals were dried with 35/.04 paper points (Schottlander), the cones coated with Total- Fill BC Sealer (Figure 5) and seated into the canals, withdrawn halfway and reseated. The coronal portion of the cones were then removed with a heated instrument and packed gently into the canal orifices (Figures 6 and 7), and the access cavity cleaned by washing with a three-in-one triple syringe. A Nayyar amalgam core was placed, the dam removed, and the occlusion checked. A final radiograph was taken (Figure 8) showing a well-condensed root canal filling in all three canals extending to length with a welladapted coronal restoration.
Discussion
The diagnosis of acute symptomatic irreversible pulpitis can sometimes be difficult; however, by repeating the patient’s sensitivity to cold, it soon became apparent which tooth was causing the trouble. The best way to treat pulpitis is to remove the inflamed tissue as quickly as possible; antibiotics have no place, as there isn’t an infection. The narrowness of the canals and the severe curvature on the mesial root can make instrumentation challenging. Sclerosis of canals takes place as a result of deposition of secondary dentin and progressive deposition of calcified masses that originate in the root pulp, according to Bernick and Nedelman (1975), and true pulp stones are made of dentin and lined by odontoblasts (Johnson and Bevelander, 1956). Pulp stones are common, ranging from 4% of first molars (Chandler, et al., 2003) to 78% of primary molars (Arys et al., 1993), and vary in size from 50 μm in diameter to several millimeters when they may occlude the entire pulp chamber (Sayegh and Reed, 1968). Therefore, if the pulp stone is not removed, the natural canal anatomy may be obscured, making shaping and disinfection difficult or impossible. Schilder reported that shaping canals is essential to endodontic success (1974), but nickel-titanium files are prone to cyclic fatigue fracture and torsional tip fracture, according to Bergmans and colleagues (2001). Glide path creation is essential when shaping with rotary nickel-titanium instruments to prevent these fractures (Patiño, et al., 2005). Ajuz and colleagues have shown that mechanical glide path preparation with Scout RaCe files has been shown to be superior to stainless steel hand files in maintaining the canal shape (2013).
As always, shaping is only part of the process of canal debridement, as reported by Byström and Sundqvist (1981); shaping and irrigating with 0.5% NaOCl significantly reduced bacterial load compared to shaping and irrigating with saline (1983), and irrigation with NaOCl and EDTA has been demonstrated to create cleaner canal walls (Baumgartner and Mader, 1987). Additionally, the use of ultrasonic irrigant activation removes more debris from canals than syringe irrigation alone (Burleson, et al., 2007).
Root canal preparation to a size 35 allows better irrigant flow and exchange (Boutsioukis, et al., 2010); creates space for the ultrasonic tip to vibrate, thereby reducing contact dampening (Ahmad, Roy and Kamarudin, 1992), which in turn improves the acoustic micro-streaming (Ahmad, Pitt Ford and Crum, 1987); and increases the reduction in bacterial load (Bhuva, et al., 2010; Carver, et al., 2007). Bioceramics (tricalcium silicates) have many uses in endodontics, due to their ability to form an apatite layer (bioactivity) and penetrate dentin tubules. Mineral trioxide aggregate (the first bioceramic) is currently employed for several endodontic techniques including root end filling, direct pulp capping, repair of perforations, and providing an apical seal in teeth with open apices (Parirokh and Torabinejad, 2010).
The literature reports several favorable properties of recently developed bioceramic sealers as root canal filling materials,including sealing ability equivalent to AH Plus® (Zhang, Li and Peng, 2009a; 2009b), low cytotoxicity (2010), antibacterial activity (2009a; 2009b), and high bond strength to dentin (Nagas, et al., 2012). It is supplied in premixed, injectable form and sets in the presence of natural canal moisture (Loushine, et al., 2011). When sealer is placed on the cone and initially seated, the canal walls are coated; withdrawing it and reseating it then allows more sealer to be placed and dispersed within the complex canal ramifications. It is imperative that the cones fit well with tug back or are customized to improve apical control (van Zyl, Gulabivala, and Ng, 2005) and that hydraulic pumping is not employed. With this technique, the GP cone acts as a carrier, and the sealer is employed to fill the entire canal space, thus providing the desired three-dimensional seal, as reported by Schilder (1967).
Conclusions
Pulp stones are common and act as a barrier to successful endo treatment. The mechanical glide path preparation with Scout RaCe files (Schottlander) allows predictable canal preparation. Single cone obturation is possible with a bioceramic sealer.
REFERENCES
1. Ahmad M, Pitt Ford TJ, Crum LA. Ultrasonic debridement of root canals: acoustic streaming and its possible role. J Endod. 1987;13(10), 490-499.
2. Ahmad M, Roy RA, Kamarudin AG. Observations of acoustic streaming fields around an oscillating ultrasonic file. Endod Dent Traumatol. 1992; 8(5): 189-194.
3. Ajuz NC, Armada L, Gonçalves LS, Debelian G, Siqueira JF Jr. Glide path preparation in S-shaped canals with rotary pathfinding nickel-titanium instruments. J Endod. 2013;39(4):534-537.
4. Arys A, Philippart C, Dourov N. Microradiography and light microscopy of mineralization in the pulp of undemineralized human primary molars. J Oral Pathol Med. 1993;22(2):49–53.
5. Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13(4):147-157.
6. Bergmans L, Van Cleynenbreugel J, Wevers M, Lambrechts P. Mechanical root canal preparation with NiTi rotary instruments: rationale, performance and safety. Status report for the American Journal of Dentistry. Am J Dent. 2001;14(5):324-333.
7. Bernick S, Nedelman C. Effect of aging on the human pulp. J Endod. 1975;1(3): 88–94.
8. Bhuva B, Patel S, Wilson R, Niazi S, Beighton D, Mannocci F. The effectiveness of passive ultrasonic irrigation on intraradicular Enterococcus faecalis biofilms in extracted single-rooted human teeth. Int Endod J. 2010;43(3):241-250.
9. Boutsioukis C, Gogos C, Verhaagen B, Versluis M, Kastrinakis E, van der Sluis LW. The effect of apical preparation size on irrigant flow in root canals evaluated using an unsteady Computational Fluid Dynamics model. Int Endod J. 2010;43(10): 874-881.
10. Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J Endod. 2007;33(7):782-787.
11. Byström A, Sundqvist G. Bacteriological evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89(4): 321-328.
12. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55(3):307-312.
13. Carver K, Nusstein J, Reader A, Beck M. In-vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molars. J Endod. 2007;33(9):1038-1043.
14. Chandler NP, Pitt Ford TR, Monteith BD. Coronal pulp size in molars: a study of bitewing radiographs. Int Endod J. 2003;36(11):757–763.
15. Bevelander G, Johnson PL. Histogenesis and histochemistry of pulpal calcification. J Dent Res. 1956;35(5):714 722.
16. Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, Pashley DH, Tay FR. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011;37(5): 673–677.
17. Nagas E, Uyanik MO, Eymirli A, Cehreli ZC, Vallittu PK, Lassila LV, Durmaz V. Dentin moisture conditions affect the adhesion of root canal sealers. J Endod. 2012;38(2): 240–244.
18. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review – Part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3): 400–413.
19. Patiño PV, Biedma BM, Liébana CR, Cantatore G, Bahillo JG. The influence of a manual glide path on the separation rate of NiTi rotary instruments. J Endod. 2005;31(2):114-116.
20. Sayegh FS, Reed AJ. Calcification in the dental pulp. Oral Surg Oral Med Oral Pathol. 1968;25(6): 873–882.
21. Schilder H. Filling root canals in three dimensions. J Endod. 1967;32(4): 281-290.
22. Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18(2):269-296.
23. Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32(2):271–275.
24. van Zyl SP, Gulabivala K, Ng YL. Effect of customization of master gutta-percha cone on apical control of root filling using different techniques: an ex vivo study. Int Endod J. 2005;38(9):658-666.
25. Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009a;35(7):1051–1055.
26. Zhang W, Li Z, Peng B. Assessment of a new root canal sealer’s apical sealing ability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009b;107(6):79–82.
27. Zhang W, Li Z, Peng B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int Endod J. 2010;43(9):769–774.
[/userloggedin]
[userloggedout][/userloggedout]
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..