Dr. Stephen R. Ottosen discusses new directions that allow endodontists to experience greater success and long-term value for patients
Introduction
The triad of bacterial-related disease in dentistry consists of coronal decay, perio-dontal infection, and endodontic infection. These have common traits, similarities, and differences. All have at their origin biofilm.
With coronal decay, the primary traits of the bacteria involved are the production of extracellular polysaccharides from sucrose to allow adhesion to the tooth surface and each other. They also digest sugars and produce lactic acid. The combination of plaque and acid leads to tooth decay.
In advanced periodontal disease, the bacteria involved primarily produce an extracellular matrix to allow adhesion to the root surface and toxins that promote inflammation of the surrounding tissue.

Figure 1: Endodontic biofilm
Figure 2: Dentin after PIPS-activated irrigation
Figure 3: Coronal biofilm byproduct: decay. Canal space biofilm byproduct: apical periodontitis.
Endodontic infections are the result of pulpal necrosis and the accompanying bacterial colonization of the pulp space. The dominant bacteria in the canal space inhabit biofilm (Figure 1) formed by the production of extracellular polymeric substances (EPS). The bacteria within the pulpal space produce various toxins. These include endotoxin (lipopolysaccharide [LPS]), exotoxin, and short chain fatty acids.
The major difference among these biofilms found in the mouth is the ability of the coronal biofilms to produce acid and physically break down the tooth structure. This results in the need for removal of destroyed enamel and dentin during treatment (restoration) of the coronal hard tissue (Figure 3).
With periodontal disease, the root surface is contaminated but generally not carious. The goal is to remove the biofilm to allow the inflammation to decrease. Further treatment of the root surface is needed to allow reattachment of the connective tissue.
In endodontic infections, the dentin and accompanying isthmuses and accessory canals are contaminated by the bacteria. These are areas that are inaccessible to endodontic files during canal instrumentation and preparation. Caries or significant demineralization or structural degradation of the dentin is not found (Figure 3). Ideal treatment would be to remove the bacteria and their associated EPS and toxins and to leave the dentin intact.
Irrigation
As 35%1 or more of the canal walls are not touched by instrumentation during endodontic treatment, we are very reliant upon irrigation to clean the pulpal space.
The following irrigation techniques generally use sodium hypochlorite 5% and EDTA 17%. Sodium hypochlorite (NaOCl) proved to be the most effective endodontic irrigant because of its excellent antimicrobial efficiency, biofilm disruption, organic tissue dissolution, and debris removal properties.2,3,4,5 EDTA, a chelator, is used during root canal therapy to remove the inorganic component of the smear layer produced by instrumentation.6 Its biofilm-dispersing property7,8 means that EDTA can also be used to “loosen” or clean endodontic biofilms. The recommended protocol for irrigation includes the use of sodium hypochlorite (NaOCl) during mechanical preparation to dissolve the organic matter and kill microorganisms followed by a chelating agent such as EDTA to remove the smear layer and to leave an adequate substrate for optimal efficacy of the final irrigant.9,10
Small gauge side-vented irrigation needle and negative pressure irrigation systems are effective in cleaning the main canal space coronal to the depth of needle placement.13 These techniques provide good columnar irrigant flow with decrease incidence of apical extrusion of irrigants into the periapical tissue.
Turbulent flow of irrigant is needed to reach anatomy that extends away from the main canal.14 Additionally, frequent exchange of irrigant assists in removing debris from the canal system adjacent to the main canals.
Sonic and ultrasonic metal and non-metal points are effective at creating turbulence, but their effectiveness is greatly diminished when they contact the canal wall.15 Upon contact with the canal wall, their energy is dampened and does not transmit efficiently through the irrigation solution. Metal ultrasonics are prone to breakage. Ultrasonics can gouge the canal wall.16
Erbium Yag (Er:YAG) lasers17 and sound wave18 irrigation activation systems are very effective (Figure 2). Both of these systems are effective due to their ability to impart energy and thus movement of the irritants within the canal system. The Fotona Erbium Yag laser performing the PIPS (Photon Induced Photo Acoustic Streaming) technique results in irrigant flow 20 to 100 times faster than ultrasonic techniques.19 PIPS is based on the activation of liquid irrigants by medium-infrared laser (2,940 Nm). The tip is placed inside the pulp chamber only. The technique uses a radial firing and stripped tip, allowing lateral emission of laser energy in the liquids. The use of subablative energy (20 mJ) delivered in a very short time (pulse duration of 50 microseconds) produces a high peak power of 400 W, causing an explosion-implosion phenomenon within the irrigant solution. The result is a strong photoacoustic shock wave that induces irrigant streaming three-dimensionally throughout the entire root canal system while avoiding any direct laser irradiation on the dentin and consequent unwanted thermal effects.11,12 The irrigant activation reaches over 20 mm away from the tip compared to only 2 mm for ultrasonics.19
Blade or “wing” type rotary irrigation instruments have shown good results in research studies.20 Easy Clean (Easy Equipa-mentos Odontologicos; Belo Horizonte, Brazil) has a blade design that is used in large angle reciprocation or complete rotation. The MicroIrrigator (MicroEndo LLC, Wenatchee, Washington) has a wing design (Figure 5) with a twist down the long axis. It is used in a reciprocating hand piece. This design provides turbulence as well as rapid slight positive and negative pressure.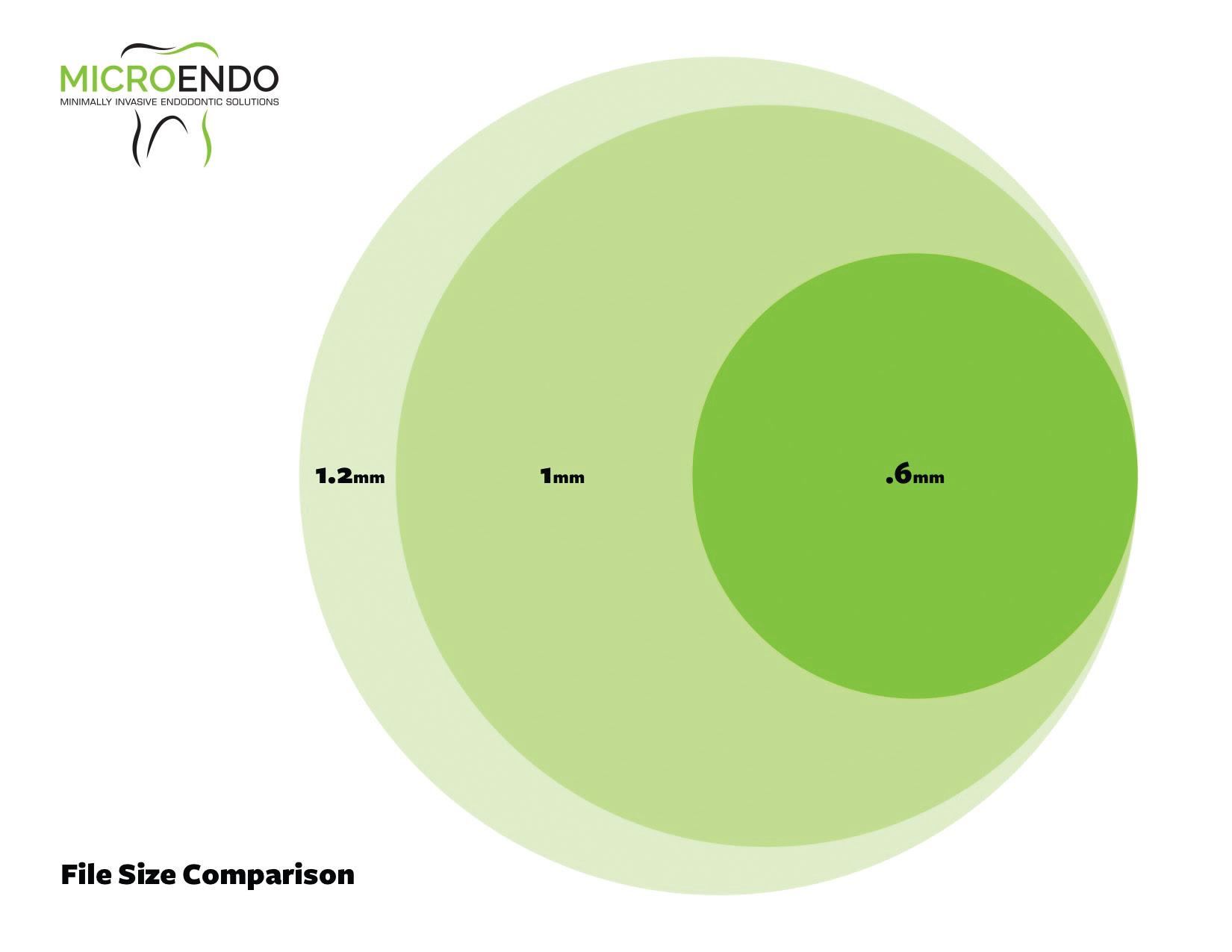
Figure 4: File cross section comparison.
Instrumentation
As instrumentation alone is ineffective at cleaning the canal system, its main function is debridement of the canal and creating, when necessary, a pathway for irrigation.
The more a canal is tapered, flared, or enlarged at the coronal portion, the weaker the root becomes.21 Rotary instruments with a taper greater than 4% (.04) have consistently shown to create small dentin defects or cracks in research studies.22,23 These cracks are suspected to lead to root fractures. The higher the torque used in rotary instrumentation, the higher the incidence of dentin cracks.24 Reciprocating techniques used with tapered files create dentin cracks at a higher incidence than a traditional rotary technique.25 This may be partially due to the large changes in file dimensions between files used in these techniques and the resulting high torques created.

Figure 5: (left to right) Komet PathGlider 20, MicroEndo MicroFile 20, Pro Taper Next X1, MicroEndo MicroIrrigator 27
The amount of dentin removed during instrumentation is directly correlated with the cross section of the rotary instruments used. The area of the circle scribed by the cross section of the instrument is found by the formula π r2. Thus, the radius of the instrument dramatically effects the amount of dentin removed during instrumentation. Most endodontic file systems have a very tapered design. This usually results in a maximum flute diameter of 1.0 mm-1.2 mm. Path Glider™ (Komet; Rock Hill, South Carolina) and MicroFiles (MicroEndo; Wenatchee, Washington) (Figure 5) have a low taper (2%-3%) design with a maximum flute diameter of 0.6 mm. When instrumenting a calcified canal, a standard rotary file may remove 200%-300% more dentin in the coronal portion of the canal (Figure 4). These small diameter files require much less torque to operate.
A recent long-term recall study of an endodontic specialty practice demonstrated less incidence of vertical root fracture in endodontically treated teeth with less tapered canal preparations.30
Finite element analysis has demonstrated that dentin preservation at the cervical area of the tooth is most important in retaining tooth strength under occlusal forces.31
Obturation
Ideally, obturation is achieved with 3D adaptation of gutta percha to the canal anatomy and a thin layer of sealer between the gutta percha (GP) and dentin.26 The only way to adapt GP to complex anatomy is through heat and pressure.27 GP only conducts heat ~4 mm ahead of a heated instrument tip. This has led to various heating techniques. Most techniques involve placing a heated metal instrument into the GP and then down packing the warmed GP with a smaller cool metal instrument. Unfortunately, the root canal has to be enlarged to accommodate the metal heating and packing instruments. These instruments rarely reach the apical area in curved or small canals to provide a closely adapted GP/sealer/dentin interface.27
Carrier-based systems have been widely used. They often do not provide a gutta-percha apical seal.28 The carrier provides a variety of challenges, and apical stripping of the warmed GP from the carrier is a common occurrence during insertion, resulting in carrier contact with the apical canal wall with no intervening GP. Mechanical (rotary) heating and compaction of gutta percha has been shown to be very effective.29
MicroObturators (MicroEndo) use a reverse wound rotary obturator (Figure 6) to place warm gutta percha into the canal and then compact the GP to form an ideal seal regardless of canal shape. The Micro-Obturators are able to obturate very small canals. If Erbium Yag (PIPS/Fotona) or multisonic (Sonendo®, Laguna Hills, California) systems are used, instrumentation is sometimes unnecessary with rotary or hand files. The MicroObturator technique uses an Obtura (Kerr, Orange, California) type backfill device to cover the obturator with warm low viscosity gutta percha (Figure 7). The obturator and GP are then coated at its apical half in sealer (Figure 8) and placed into the canal 1 mm from the apical foramen (Figure 9). The obturator is then rotated at 20,000 rpm and withdrawn from the canal. This results in the GP on the rotary instrument being adapted to the canal of the root, and an ideal seal results.
Figure 6: Irrisonic tip and Easy Equipmentos Odontologicos Easy Clean irrigator
Figure 8: Applying sealer to warm gutta percha
Figure 7: MicroObturator being coated in warm gutta percha
Figure 9: MicroObturator placed to working length (1 mm short of apical foramen)
Cases
Case 1 (Figures 10-12)
Tooth No. 30 was diagnosed with pulpal necrosis and chronic apical periodontitis. The canal anatomy was uncomplex with one broad distal canal and two mesial canals that joined. Very little root dentin was removed to debride the canals. A size 20 .03 PathGlider (Komet) was used as the final instrument. The canal system was cleaned with Er-Yag laser-activated irrigation (Fotona). Obturation was completed with a size 16 MicroObtuator (MicroEndo), NanoFlow (Healthdent Technology Intl., Inc.; Palmdale, California) gutta percha and Kerr Pulp Canal Sealer EWT. Ideal healing was seen at 1-year follow-up. 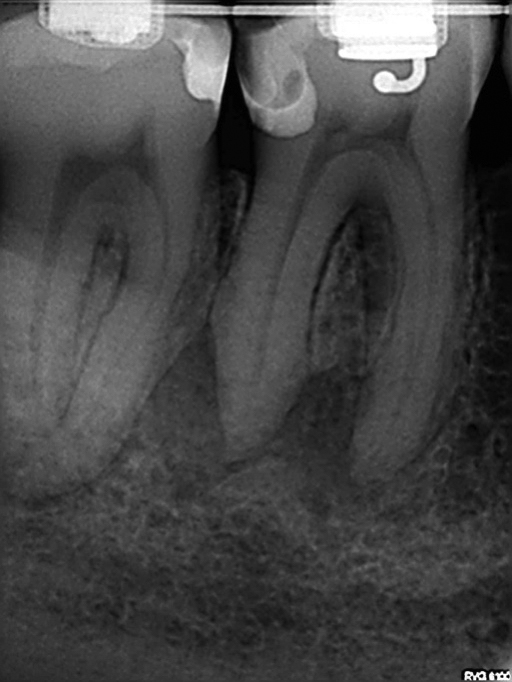
Figure 10: Case 1 — pre-op radiograph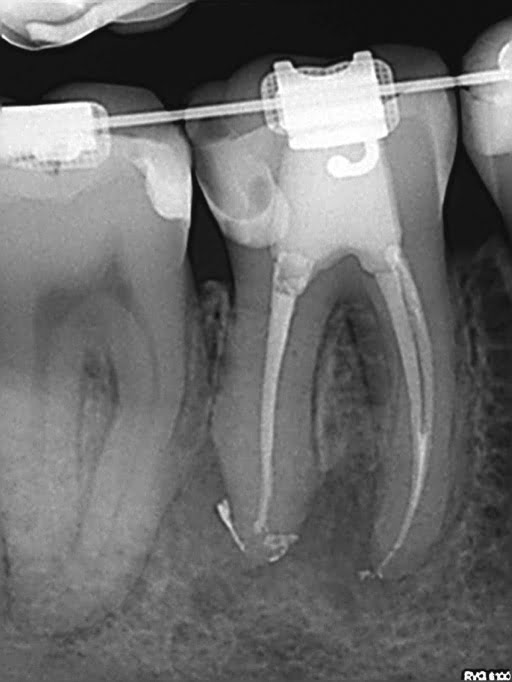
Figure 11: Case 1 — post-op radiograph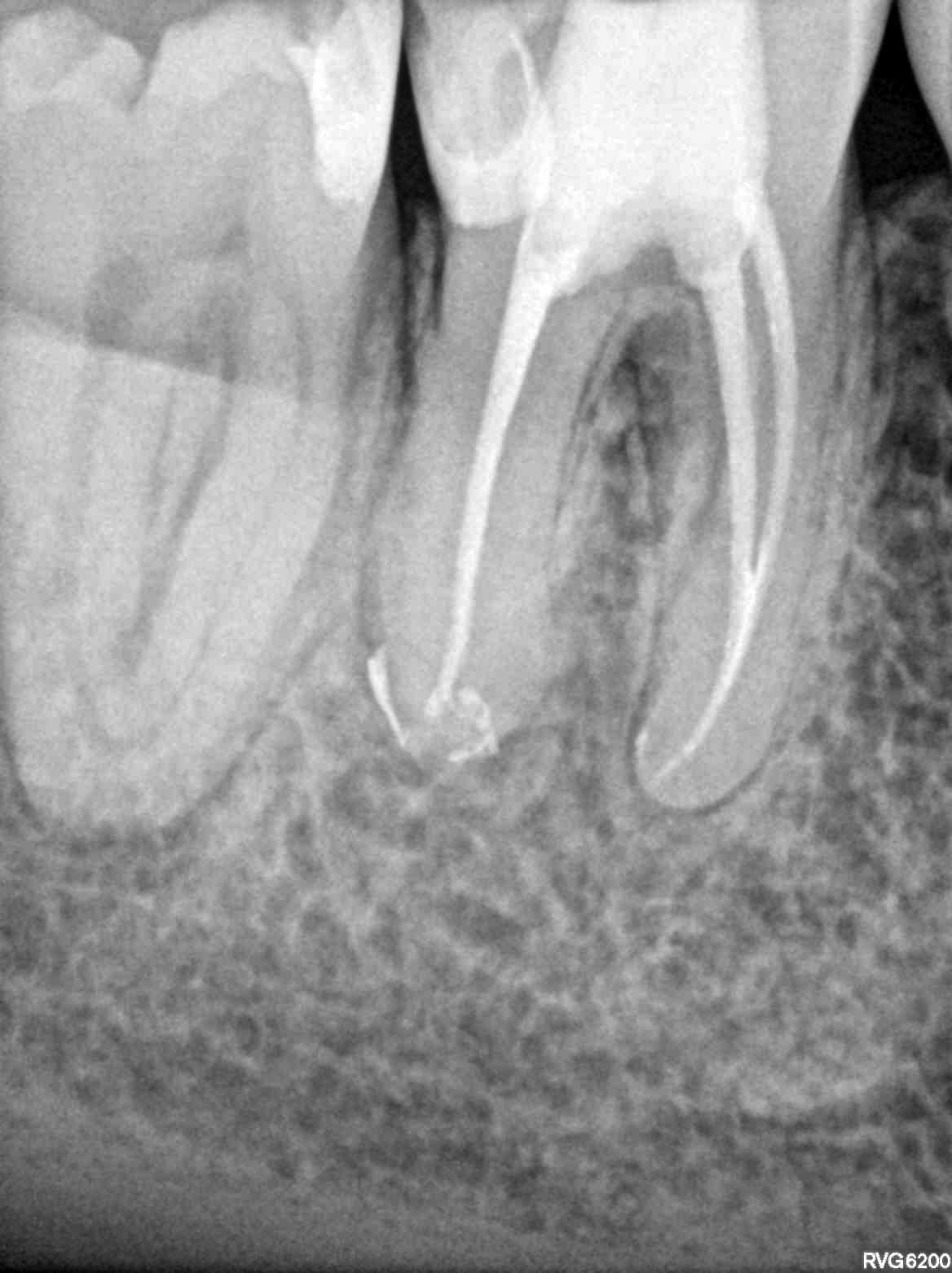
Figure 12: Case1 — 1-year recall radiograph
Case 2 (Figures 13-14)
Tooth No. 4 was diagnosed with pulpal necrosis and acute apical periodontitis. After access, No. 4 was irrigated with PIPS, dried with paper points, and obturated with a MicroObturator size 16. NanoFlow gutta percha and Kerr EWT sealer were used. No instrumentation was needed due to the size of the existing canal space.

Figure 13: Case 2 — pre-op radiograph
Figure 14: Case 2 — post-op radiograph
Case 3 (Figures 15-16)
Tooth No. 3 was diagnosed with pulpal necrosis and acute apical periodontitis. The palatal canal was instrumented to a size 35 .02 MicroFile (MicroEndo); the mesial and distal buccal canals to a size 30.02 MicroFile. Irrigation was completed with Er-Yag laser-activated irrigation. Obturation was completed with a MicroObturator size 16. NanoFlow gutta percha and Kerr EWT sealer were used.

Figure 15: Case 3 — pre-op radiograph
Figure 17: Cracked mesial root No. 18
Figure 16: Case 3 — post-op radiograph
Figure 18: No. 31 endodontically treated with minimally invasive techniques
Summary
For the past 30-plus years, the techniques associated with endodontic treatment have been focused on creating tapered canals to allow adequate cleansing and obturation. With new irrigation and obturation techniques, creating these tapers and the associated tooth weakening is no longer necessary (Figure 17). Preservation of cervical tooth structure maintains the strength of the area of the tooth, which under occlusal loading has the highest stress. Thus, conservative instrumentation in the cervical area of the canals does not weaken the tooth as may happen with more aggressive instrumentation (Figure 18).
We are at an amazing time when new techniques, technology, and understanding allow us to step away from previously held beliefs and perceptions. We can move in new directions that will allow us to experience greater success and long-term value for our patients and ourselves. Most importantly, the time is now!
- Peters OA, Schonenberger K, LaiB A. Effects of four NiTi preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34(3):221-230.
- Waltimo T, Trope M., Haapasalo M, Řrstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J Endod. 2005;31(12): 863–866.
- Zehnder M. Root canal irrigants. J Endod. 2006;32(5): 389-398.
- Del Carpio-Perochena AE, Bramante CM, Duarte MA, et al. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod. 2011;37(8):1134-1138.
- Arias-Moliz MT, Ordinola-Zapata R, Baca P, et al. Antimicrobial activity of chlorhexidine, peracetic acid and sodium hypochlorite/etidronate irrigant solutions against Enterococcus faecalis biofilms. Int Endod J. 2015;48(12):1188-1193.
- Teixeira CS, Felippe MCS, Felippe WT. The effect of application time of EDTA and NaOCl on intracanal smear layer removal: an SEM analysis. Int Endod J. 2005;38(5):285-290.
- Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72(3):2064-2069.
- Cavaliere R, Ball JL, Turnbull, L, Whitchurch CB. The biofilm matrix destabilizers, EDTA and DNaseI, enhance the susceptibility of nontypeable Hemophilus influenzae biofilms to treatment with ampicillin and ciprofloxacin. Microbiologyopen. 2014;3(4):557-567.
- Zehnder M. Root canal irrigants. J Endod. 2006;32(5): 389-398.
- Baca P, Junco P, Arias-Moliz MT, González-Rodríguez MP, Ferrer-Luque CM. Residual and antimicrobial activity of final irrigation protocols on Enterococcus faecalis biofilm in dentin. J Endod. 2011;37(6):363-366.
- Lin S, Liu Q, Peng Q. et al. The ablation threshold of Er:YAG laser and Er,Cr:YSGG laser in dental dentin. Sci Res Essays. 2010;5(16):2128-2135.
- Majaron B, Lukac M, Sustercic D, Funduk N, Skaleric U. Threshold and efficiency analysis in Er:YAG laser ablation of hard dental tissue. Proc SPIE. 1996;2922:233-242.
- Siu C, Baumgartner JC. Comparison of the debridement efficiency of the EndoVac irrigation system and conventional needle root canal irrigation in vivo. J Endod. 2010;36(11): 1782-1785.
- Neelakantan P, Devaraj S, Jagannathan N. Histologic assessment of debridement of the root canal isthmus of mandibular molars by irrigation activation techniques ex vivo. J Endod. 2016;42(8):1268-1272.
- Amato M, Vanoni-Heineken I, Hecker H, Weiger R. Curved versus straight root canals: the benefit of activated irrigation techniques on dentin debris removal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(4):529-534.
- Retsas A, Koursoumis A, Tzimpoulas N, Boutsioukis C, Uncontrolled removal of dentin during in vitro ultrasonic irrigant activation in curved root canals. J Endod. 2016;42(10):1545-1549.
- Lloyd A, Uhles J, Clement D, Garcia-Goday F. Elimination of intracanal tissue and debris through a novel laser-activated system assessed using high-resolution micro-computed tomography: a pilot study. J Endod. 2013;40(4):584-587.
- Molina B, Glickman G, Vandrangi P, Khakpour M. Evaluation of root canal debridement of human molars using the GentleWave system. J Endod. 2015;41(10):1701-1705.
- Kooh J, Jaramillo D, DiVito E, Peters O. Irrrigant flow during photon-induced photoacoustic streaming (PIPS) using Particle Image Velocimetry (PIV). Clin Oral Investig. 2016;20(2):381-386.
- Kato AS, Cunha RS, da Silveira Bueno CE, et al. Investigation of the efficacy of passive ultrasonic irrigation versus irrigation with reciprocating activation: an environmental scanning electron microscopic study. J Endod. 2016;42(4):659-663.
- Rundquist B, Versluis A. How does taper effect root stresses? Int. Endod J. 2006;39(3):226-237.
- Bier C, Shemesh H, Tanomaru-Filho M, Wesselink P, Wu M. The ability of different nickel-titanium rotary instruments to induce dentinal damage during canal preparation. J Endod. 2009;35(2):236-238.
- Li SH, Lu Y, Song D, et al. Occurrence of dentinal microcracks in severely curved root canals with ProTaper Universal, WaveOne, and ProTaper Next file systems. J Endod. 2015;41(11):1875-1879.
- Dane A, Capar I, Arslan H, AkçayM, Uysal B. Effect of different torque settings on crack formation in root dentin. J Endod. 2016;41(2):304-306.
- Burklein, Tsotsis P, Schäfer E. Incidence of dentinal defects after root canal preparation: reciprocating versus rotary instrumentation. J Endod. 2013;39(4):501-504.
- Wu M, Wesselink P, Boersma J. A 1-year follow-up study on leakage of four root canal sealers at different thicknesses. Int Endod J. 1995;28(4):185-189.
- Smith RS, Waller RN, Loushine RJ, Kimbrough WF. Effect of varying the depth of heat application on the adaptability of gutta percha during warm vertical compaction. J Endod. 2000;26(11):668-672.
- Juhlin JJ, Walton RE, Dovgan JS. Adaptation of Thermafil components to canal walls. J Endod. 1993;19(3):130-135.
- O’Neill K, Pitts D, Harrington G. Evaluation of the apical seal produced by the McSpadden compactor and by lateral condensation with chloroform softened primary core. J Endod. 1983;9:190-197.
- Grover, RE. Observation from follow-ups of intermediate to long term endodontically treated teeth. Presented at: AAE Annual Meeting. New Orleans, Louisiana; 2017.
- Grande NM, Plotino G, Sinibaldi R, Mangani F, Cerutti A, Gambarini G. UC-based finite element analysis study on the stress distribution in root canal treated teeth with different cavity access and root canal preparation geometry. Presented at: AAE Annual Meeting. Seattle, Washington; 2015.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Stephen R. Ottosen, DDS, MSD, graduated from the University of Washington School of Dentistry in 1994 and the University of Washington Endodontic Specialty Training in 1996. His research was in NiTi rotary instruments. He opened his practice in 1996 in Wenatchee, Washington. Dr. Ottosen has taught for the University of Washington continuing education department. He provides endodontic continuing education to several Washington state study clubs and dental societies. Dr. Ottosen is a member of the American Dental Association, the American Association of Endodontists, and the North Central District of the American Dental Association.
Stephen R. Ottosen, DDS, MSD, graduated from the University of Washington School of Dentistry in 1994 and the University of Washington Endodontic Specialty Training in 1996. His research was in NiTi rotary instruments. He opened his practice in 1996 in Wenatchee, Washington. Dr. Ottosen has taught for the University of Washington continuing education department. He provides endodontic continuing education to several Washington state study clubs and dental societies. Dr. Ottosen is a member of the American Dental Association, the American Association of Endodontists, and the North Central District of the American Dental Association.