Continuing his series on endodontics, Dr. Tony Druttman looks at an important type of disinfection
The primary goal of root canal treatment is to remove pulp tissue, necrotic debris and bacteria from the root canal system. As I have explained in earlier issues, the canal anatomy of many teeth is very complex, and studies have shown that the canal system cannot be disinfected by mechanical instrumentation alone.1,2 Irrigants have therefore have to be used in conjunction with mechanical preparation for the following purposes:
- Removal of debris created during instrumentation
- Lubrication
- Elimination of microorganisms
- Dissolution of soft tissue
- Removal of smear layer
 There are a number of different irrigants available and several different techniques for their delivery. No one irrigant can satisfy all the demands, and therefore, an optimal irrigation regime advocates the use of different irrigants in sequence.3 There are many factors that affect the effectiveness of irrigation, including canal anatomy, irrigating solution, concentration and the technique of delivery. Although water and local anesthetics are often used, these have no chemical effect on the canal contents, and their only role is to flush away debris created during mechanical preparation. Regrettably, they are often chosen by clinicians who do not use a rubber dam. Although there are several different irrigants on the market, with new ones being developed, sodium hypochlorite and EDTA continue to be among those most widely recommended.4
There are a number of different irrigants available and several different techniques for their delivery. No one irrigant can satisfy all the demands, and therefore, an optimal irrigation regime advocates the use of different irrigants in sequence.3 There are many factors that affect the effectiveness of irrigation, including canal anatomy, irrigating solution, concentration and the technique of delivery. Although water and local anesthetics are often used, these have no chemical effect on the canal contents, and their only role is to flush away debris created during mechanical preparation. Regrettably, they are often chosen by clinicians who do not use a rubber dam. Although there are several different irrigants on the market, with new ones being developed, sodium hypochlorite and EDTA continue to be among those most widely recommended.4
Sodium hypochlorite
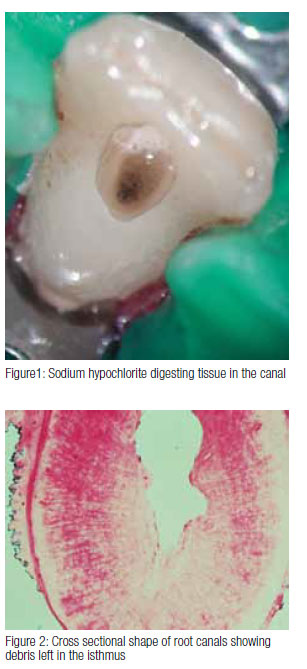
Sodium hypochlorite can be used in different concentrations, and some research has shown that while there is no difference in the antimicrobial effect between 0.5% and 5%,2 there is significant difference in tissue dissolving capability, which is also dependant on volume, contact time, and surface area.5 Recent research has shown that 5.25% sodium hypochlorite is more effective in removing bacteria from the dentinal tubules than lower concentrations.6 Heating sodium hypochlorite also enhances its tissue dissolving capability.7 Because there are many uninstrumented areas left during mechanical preparation, we depend on the irrigant to dissolve and flush away the debris left behind (Figure 1).
While bacteria will often exist in planktonic form (in suspension) in the root canals, they may also adhere to the canal walls in the form of biofilms. These are complex communities of bacteria imbedded in a polysaccharide matrix. Sodium hypochlorite is the most effective irrigant for use against biofilms.8
Much of the canal contents can be removed by mechanical instrumentation, and this is particularly the case where the prepared canal shape matches the size and shape of the instruments used in the preparation of the canal. Unfortunately, this is rarely the case. Research has shown that many canals are not circular in cross section (Figure 2), and that the development of an optimized canal shape will often over prepare some areas of the canal while leaving others completely untouched by instrumentation.9 While much of the debris is brought out of the canal within the flutes of the rotating instruments, some is left behind in the fins, isthmuses, and extremities of the canal. This is borne out both by clinical evidence and by research.10 Looking through the microscope into a prepared canal, one can often see debris left behind.
 The chlorine in sodium hypochlorite is responsible for dissolving organic tissue, but it tends to get used up very rapidly according to published research. Therefore regular and frequent irrigant replacement is required. The term “copious irrigation” is one often used, suggesting that large volumes of irrigant should be delivered into the canal. How the irrigant is delivered into the canal and how it is agitated is more important than the actual volume used.11
The chlorine in sodium hypochlorite is responsible for dissolving organic tissue, but it tends to get used up very rapidly according to published research. Therefore regular and frequent irrigant replacement is required. The term “copious irrigation” is one often used, suggesting that large volumes of irrigant should be delivered into the canal. How the irrigant is delivered into the canal and how it is agitated is more important than the actual volume used.11
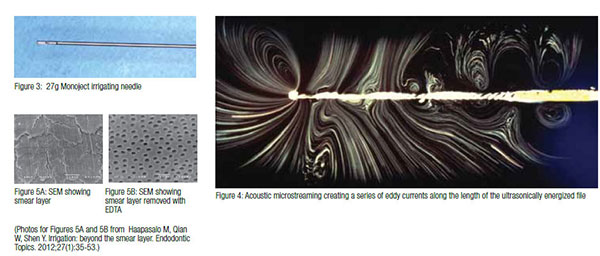
A common form of hand irrigation is with a 27g needle with a side vent that allows the irrigant to flow towards the access cavity rather than being forced through the apex (Figure 3). The 27g needle diameter is equivalent to a size 40 hand file. This means that unless the canal is prepared to at least a size 40, the needle will not reach the apex, and however much irrigant is delivered, the debris will never be flushed out of the apical few millimeters unless a different strategy is used.
Irrigant exchange can be improved in a number of ways, including pressure alteration, sonics, and ultrasonics. The simplest is to pump hand files in the canal and if using rotary files, this happens naturally as the files are moved up and down the canal. A recent development of the hand file technique is the manual dynamic agitation technique in which the obturating gutta-percha cone is pumped up and down in the canal. This creates alternating pressure and vacuum and has been shown to allow irrigants to enter lateral canals and is particularly effective in curved canals.12 Another very effective technique is to use sonic and ultrasonic irrigation. Utrasonic activation uses an energized file to dislodge debris from the canal walls into the suspension. Research has show that this can be a very effective way of cleaning areas such as the isthmus of debris.13 It does so by creating turbulent flow and acoustic microstreaming along the energized file (Figure 4).
The challenge is always to remove debris that has been compacted into stagnation areas of the canal wall. While sodium hypochlorite is very effective at dissolving organic tissue when there is a large surface area of contact, when the contact surface is minimal, it is difficult for the irrigant to penetrate through the plug of material. An ultrasonically energized file directed against the plug will effectively remove it. IrriSafe™ (Satelec Acteon) ultrasonic needles have been developed for passive ultrasonic irrigation and have the advantage that they cannot cut into the canal wall.
EDTA
Ethylene diamine tetracetic acid (17%) is used to dissolve the inorganic material within the root canal. During preparation, smear layer forms on the wall of the prepared canal, and this may harbor bacteria. Sodium hypochlorite and EDTA counter the effect of each other so EDTA should only be used as the penultimate rinse. EDTA gel acts as a lubricant for the files and can be used in narrow canals to reduce the frictional resistance of the canal. Once preparation has been competed, the canal should be rinsed with sodium hypochlorite and the irrigant exchanged using either active or passive irrigation. The same is done with EDTA to remove the smear layer and expose the dentinal tubules. The hypochlorite rinse is then repeated to remove residual microbes from the canal walls, and the canal is given a final rinse with water.
Caution
Irrigants such as these should be used with caution when using irrigating needles to avoid accidents such as injection through the apex. The smaller the needle diameter and the closer the irrigating needle can be placed to the apex of the canal, the less space there is for the irrigant to flow coronally and the greater the pressure required to create flow. This means that it is quite possible to inject the irrigant beyond the confines of the canal and into the periapical tissues. This can have dramatic and painful consequences for the patient. It is therefore important that the needle is loose in the canal and that the force on the plunger is just sufficient for the irrigant to flow.
Conclusion
Sodium hypochlorite and EDTA are among the most effective irrigants that are available in endodontics. As these are not inert substances, they have to be used safely and should always be used with a rubber dam.
Next issue: Preparation techniques
- Baker NA, Eleazer PD, Averbach RE, Seltzer S. Scanning electron microscopic study of the efficacy of various irrigating solutions. J Endod. 1975;1(4):127-135.
- Byström A, Sundqvist G. Bacteriological evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89(4):321-328.
- Basrani B, Haapasalo M. Update on endodontic irrigating solutions. Endodontic Topics. 2012; 27(1):74-102.
- Haapasalo M, Qian W, Shen Y. Irrigation: beyond the smear layer. Endodontic Topics. 2012;27(1):35-53.
- Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4(2):60-64.
- Berber VB, Gomes BP, Sena NT, Vianna ME, Ferraz CC, Zaia AA, Souza-Filho FJ. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int Endod J. 2006;39(1):10-17.
- Cunningham WT, Balekjian AY. Effect of temperature on collagen-dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1980;49(2):175-177.
- Clegg MS, Vertucci FJ, Walker C, Belanger M, Britto LR. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. J Endod. 2006;32(5):434-437.
- Peters OA, Laib A, Göhring TN, Barbakow F. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J Endod. 2001;27(1):1-6.
- Endal U, Shen Y, Knut A, Gao Y, Haapasalo M. A high-resolution computed tomography study of changes in root canal ithmus area by instrumentation and root filling. J Endod. 2011;37(2):223-227.
- Druttman AC, Stock CJ. An in vitro comparison of ultrasonic and conventional methods of irrigant replacement. Int Endod J. 1989;22(4):174-178.
- Bronnec F, Bouillaguet S, Machtou P. Ex vivo assessment of irrigant penetration and renewal during the final irrigation regimen. Int Endod J. 2010;43(8):663-672.
- Susin L, Liu Y, Yoon JC, Parente JM, Loushine RJ, Ricucci D, Bryan T, Weller RN, Pashley DH, Tay FR. Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed system. Int Endod J. 2010;43(12):1077-1090.
Stay Relevant With Endodontic Practice US
Join our email list for CE courses and webinars, articles and more..

 Tony Druttman, MSc, BChD, BSc, is an endodontist working in central London. He is also a part-time teacher at the Eastman Dental Institute, University of London, and lectures in the UK and abroad.
Tony Druttman, MSc, BChD, BSc, is an endodontist working in central London. He is also a part-time teacher at the Eastman Dental Institute, University of London, and lectures in the UK and abroad.
