CE Expiration Date:
CEU (Continuing Education Unit): Credit(s)
AGD Code:
Educational aims and objectives
The aim of this article is to compare the efficacy of EndoActivator®, Eddy polyamide tips, XP-endo Finisher, and conventional needle irrigation in the removal of debris and smear layer from root canals walls.
Expected outcomes
Endodontic Practice US subscribers can answer the CE questions by taking the quiz to earn 2 hours of CE from reading this article.
Take the quiz by correctly answering the questions will demonstrate the reader can:
- Realize that whatever technique or device is used for final irrigation, agitation of the irrigating solution should always be performed in order to improve the effectiveness of debris and smear layer removal.
- Recognize the importance of an effective irrigation protocol.
- Realize some drawbacks to the conventional needle irrigation technique.
- Identify some characteristics of EndoActivator, Eddy, and XP-endo Finisher.
Since smear layer removal is essential for endodontic success, this article comparing three agitation devices can offer insights into which technique best suits individual circumstances. Read what Drs. Sandra Tipanta, Osvaldo Zmener, and Cornelis H. Pameijer have learned from their study.
Drs. Sandra Tipanta, Osvaldo Zmener, and Cornelis H. Pameijer investigate the effectiveness of three different irrigation/agitation techniques (EndoActivator®, Eddy, and XP-endo Finisher) at removing debris and the smear layer from root canal systems
Complete removal of debris (DE) and the smear layer (SL) from the root canal system is an essential step for endodontic success (Schafer and Zapke, 2000; Paque, et al., 2011).
Although during root canal preparation the operator relies foremost on endodontic instruments, an effective irrigation protocol is of critical importance as it cleans the root canal system from the SL, flushes out DE, and acts as a tissue solvent, bactericidal agent, and lubricant.
The use of a sodium hypochlorite solution (NaOCl) removes loosely attached DE and organic material, while chelating agents such as ethylenediaminetetraacetic acid (EDTA) are necessary to effectively remove the inorganic part of the SL (Hülsmann, et al., 1997; Svec and Harrison, 1977). Historically, different protocols have been recommended for root canal irrigation, but all have demonstrated that some amount of DE and the SL remained on the canal walls after instrumentation and irrigation (Svec and Harrison, 1977; Haapasalo, et al., 2014; Zehnder, 2006).
The use of a conventional needle irrigation technique is the traditional method of delivering irrigating solutions to the root canals. However, when using this technique, solutions do not always come into contact with all irregularities of the root canal walls (Schafer and Zapke, 2000; Paque, et al., 2011; Blank-Gonçalves, et al., 2011).
To improve the efficacy of irrigation, agitation of the irrigating solution has been proposed (Blank-Gonçalves, et al., 2011; Caron, et al., 2010). Among these techniques, the EndoActivator® (Dentsply Sirona) was found to improve, to some extent, the effectiveness of irrigation. The EndoActivator is a cordless battery-activated sonic handpiece that uses non-cutting polymer tips for a safe intracanal agitation of the irrigation solutions. The tips are available in three different sizes, producing 2,000 to 10,000 cycles per minute.
The instrument produces a three-dimensional movement with high-amplitude acoustic oscillations (Gu, et al., 2009). It was also recommended to use the EndoActivator after the cleaning and shaping of canals (Uroz-Torres, et al., 2010).
Eddy® (VDW) is another sonic device that activates irrigating solutions by means of a 25/.04 smooth flexible non-cutting polyamide tip, powered by a sonic scaler at a frequency of 5,000 to 6,000 cycles per minute. Eddy (EPt) produces an acoustic streaming, which effectively removes DE and the SL that adhere to root canal walls, including in difficult-to-access areas of the canal system (Khaord, et al., 2015; Urban, et al., 2017; Bayari, et al., 2017).
More recently, the XP-endo Finisher (FKG Dentaire SA) has also been introduced for the activation of irrigants. The XP-endo Finisher (XPe) consists of a size 25/.00 non-tapered instrument constructed of MaxWire martensite-austenite electro-polish-flex alloy (FKG Dentaire SA) (Bao, et al., 2017), which is a special nickel-titanium alloy capable of producing an expanding movement when the instrument is used at body temperature (35°C-37°C). Under these conditions, the instrument can adapt three-dimensionally to the canal anatomy. To be effective, the XP-endo Finisher must be used at 800 rpm and, after canal preparation, to a size 25 or larger (Bao, et al., 2017).
The purpose of this in vitro SEM study was to compare the efficacy of Endoactivator, Eddy, and XP-endo Finisher in the removal of DE and the SL from root canal walls as a final step in irrigation. Conventional needle irrigation technique served as the control.
The null hypothesis was that there would be no significant differences among the tested irrigation systems in their efficacy to remove DE and the SL from the root canal walls.
Materials and methods
The protocol of this study was approved by the Ethics Committee of the Argentina Dental Association (protocol 0119/18). Forty (n = 40) extracted human lower premolars with single straight root canals were used. They were stored at 4°C in 0.1% thymol solution before being treated. The teeth were decoronated to a standardized length of 18 mm.
After the removal of gross pulpal tissues, the working length (WL) was established by advancing a size 10 K-file into the canal until just visible at the apex under a stereomicroscope (Carl Zeiss, Oberkochen, Germany) followed by subtracting 1 mm. All teeth were assigned to four groups of 10 teeth each (n = 10), using a stratified sampling method to ensure that all groups had teeth of similar average dimensions. This was accomplished by the use of the Minitab Statistical Analysis Package (Minitab 10.1, Minitab Inc., State College, Pennsylvania).
In all groups, the canals were prepared with ProTaper Next™ NiTi instruments (Dentsply Sirona, Tulsa, Oklahoma), strictly according to the manufacturer’s recommendations. Biomechanical preparation of the apical part of the canals was considered complete when an instrument No. X3 (equivalent to a size No. 30/.07 taper) could easily be inserted to the WL while maintaining apical patency. Throughout preparation, the canals were irrigated with 5 ml of 5.25% NaOCl before and after each instrument using a 30-guage NaviTip™ needle (Ultradent Products, Inc, South Jordan, Utah) placed 1 mm short of the WL without binding. After preparation, the canals of each group were subjected to different final irrigation/agitation techniques — Group 1: EAc; Group 2: PTe; Group 3: XPe; Group 4: CNi.
To ensure retention of the irrigants in the canal space, the root apex was sealed with cyanoacrylate (La Gotita, Buenos Aires, Argentina) taking care to prevent getting cyanoacrylate in the canal. The final irrigation/activation procedures were performed according to the following protocols:
Group 1: EAc
(n = 10): 2 ml of 5.25% NaOCl was injected with a No. 30-gauge NaviTip needle (Ultradent Products, Inc.) and the solution activated for 30 seconds with a No. 25/.04 non-cutting polymer tip powered to 10,000 cycles per minute. The tip was used with in-and-out movements of approximately 6 mm-8 mm to the full WL. After flushing with saline, the irrigation/activation procedure continued by filling the canals with 17% EDTA and agitation with EAc for another 30 seconds. The cycle of irrigation/activation with 5.25% NaOCl was repeated, and the canals were finally flushed with 5 ml of 5.25% NaOCl followed by rinsing with copious amounts of saline and dried with capillary suction tips and paper points. The polymer tip was discarded after each use.
Group 2: EPt
(n = 10): The procedure was similar to that used in Group 1; however, the agitation was performed with EPt using a No. 20/.02 non-cutting polymer tip powered at a frequency of 6000 Hz by an air scaler (NSK AS2000; NSK, Japan) set at maximum speed. The Eddy tip was discarded after each use.
Group 3: XPe
(n = 10): A similar procedure was used as in to Group 1 and 2, but agitation was performed with the XPe activated at 800 rpm in a torque controlled contra-angle. To control the proper length a rubber stop was used at a predetermined length. The XPe was also discarded after each use.
Group 4: CNi
(n = 10): After instrumentation, a No. 30-gauge NaviTip needle was placed 1 mm short of the WL and the canal rinsed with 3 ml of 5.25% NaOCl. The irrigating solution was left in place for 30 seconds, evacuated, and followed by 3 ml of 17% EDTA for 30 seconds. This cycle of irrigation was repeated, and the canals were finally flushed with 5 ml of 5.25% NaOCl followed by rinsing with copious amounts of saline. They were then dried with capillary suction tips and paper points. The needle was used for one canal only.
Irrigation for all groups was performed in a temperature-controlled environment of 37°C. The access openings were sealed with Cavit™ (3M ESPE, Seefeld, Germany) and the samples coded to allow for a blinded evaluation. In preparation for scanning electron microscopy, a groove was prepared on the buccal and lingual root surface with a diamond disc under copious water cooling, making sure not to penetrate the root canal space. The samples were then immersed in liquid nitrogen and split longitudinally with a mallet and chisel. Note the seal of the access openings prevented the liquid nitrogen from penetrating the root canal.
Teeth with evidence that the groove had penetrated into the canal space or that exhibited an irregular cleavage were discarded and replaced with new specimens. The paired halves of each tooth were mounted side by side on an aluminium stub, coated with gold-palladium and examined in a scanning electron microscope (JEOL JSM 6490LV, Tokyo, Japan) operated at 15.5 Kv. Serial SEM photomicrographs were made at 1000x magnification and aligned in such a manner that they generated a horizontal panoramic view covering the total circumference of the canal walls at levels of 1 mm, 5 mm, and 10 mm from the WL. Digital images were transferred to a computer and analyzed, using Image-Pro® plus 6.0 software (Media Cybernetics, Bethesda, Maryland).
For evaluation, a slight modification of the method described by Mayer, et al. (2002), was used. Briefly, a 300-micron square grid was superimposed on the photographs, and a determination was made as to the presence of DE and SL remnants. Each square was considered as an assessment unit, and the examination space was set at 600 microns square.
The total area of each of the experimental levels was analyzed by two evaluators who were blinded to the experimental groups and who independently scored the presence of DE and SL. Before the analysis, both evaluators were calibrated by having them analyze a set of SEM photomicrographs at a magnification of 1000x obtained from the canal walls of teeth subjected to endodontic instrumentation and different irrigation regimens. Inter-observer reproducibility was measured by the Kappa coefficient.
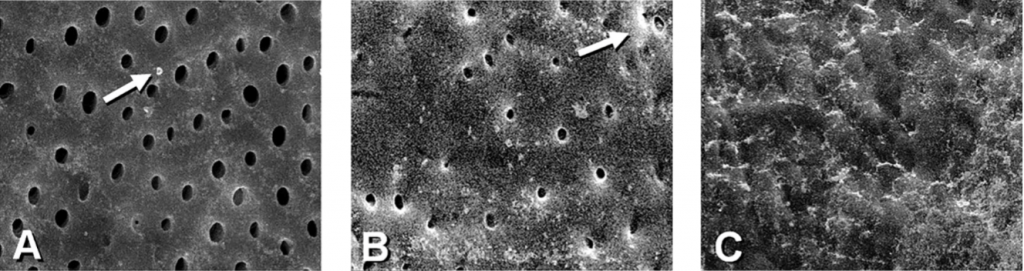
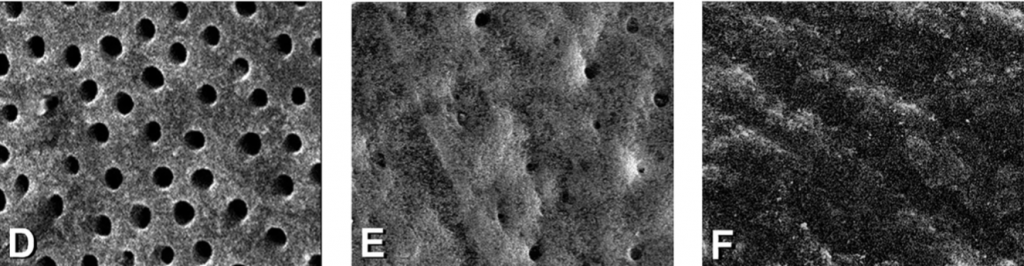
The amount of DE and SL layer in each measuring unit was assessed based on a score of 1-3. For DE, a Score 1 was assigned when no DE or isolated small particles were present. Score 2 indicated that DE covered more than 30% of the canal walls, and a Score 3 indicated that DE covered the entire canal wall. For the SL, a score 1 was assigned when a regular pattern of open dentinal tubules and no SL was present. Score 2 indicated that some dentinal tubules were open, and the others were covered by SL. Score 3 was assigned when a continuous SL covered the canal walls, and no dentinal tubules were seen.
Statistical analysis
The average score of each level was calculated by dividing the sum of all individual scores by the number of evaluation units. Mean scores for DE and SL were then calculated for each tooth and for each group and statistically analyzed for significance between groups using the Kruskal-Wallis non-parametric Anova and Dunn’s tests. The results obtained at each evaluation level within each group were analyzed using the Friedman test and Tukey’s multiple comparison test. The level of significance was set at p <0.05.
Results
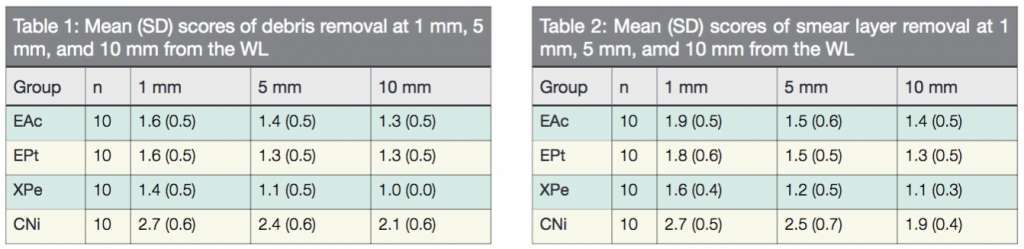
The inter-observer reproducibility was 94%, which constituted a strong agreement. Therefore, the scoring of the samples was considered reliable. The mean scores of DE and SL recorded at 1 mm, 5 mm, and 10 mm from the WL are listed subsequently Tables 1 and 2. Figure 1 is a representative image of scores 1, 2, and 3 for DE and SL, respectively. In all groups, SEM observation showed cleaner canals at 5 mm and 10 mm compared to 1 mm from the WL.
Comparison between groups showed that at all evaluation levels, the canals in which the irrigants were agitated with either EndoActivator, Eddy, or XP-endo Finisher had significantly less surface DE and SL than those in which the control was used. XP-endo Finisher removed significantly more DE and SL than EndoActivator and Eddy, while no significant differences were observed between EndoActivator and Eddy. Based on these results, the null hypothesis was rejected.
Discussion
This study evaluated the cleanliness of root canals after the use of different irrigation/agitation systems. After root canal preparation, remnants of DE or the SL may prevent the adaptation of filling materials to the root canal walls, thus jeopardizing their sealing properties. Therefore, DE and SL remnants should be totally removed before canal obturation.
As per protocol, a combination of NaOCl and EDTA solutions were used during root canal instrumentation, as well as for the final irrigation/agitation step. This combination of irrigants was found to be one of the more effective irrigation regimes to produce clean root canal walls (Baumgartner and Mader, 1987; Hülsmann, et al., 1997; Mayer, et al., 2002).
However, several studies (Schafer and Zapke, 2000; Peters and Barbakow, 2000; Ahlquist, et al., 2001) have demonstrated that none of the irrigants and irrigation techniques are totally efficient in completely cleaning the irregular areas of canal walls. For that reason, an additional irrigation/agitation step has been proposed (Duque, et al., 2017). In this study EndoActivator, Eddy, and XP-endo Finisher irrigation/agitation techniques were tested, while a conventional needle irrigation technique was used as control since the last, a no-agitation method, is still frequently used in endodontic treatment.
The results reported here are in support of Caron, et al. (2010), Jiang, et al. (2012), Kumar, et al. (2015), Mendonça, et al. (2015), Bayari (2017), demonstrating that improved DE and SL removal is accomplished with supplementary irrigation/agitation techniques.
Previous studies (Peters and Barbakow, 2000; Paque, et al., 2006) have shown that regardless of the instrument or instrumentation technique, the most apical part of the root canal is the least clean. Findings by Abou-Rass and Piccinino (1982) have shown that instruments used for irrigation have to be in close proximity to the root canal walls in order for irrigants to be effective.
In the present study, the 30-gauge needle used for the final step of irrigation in canals prepared to a size 30/.07 did not allow the irrigants to reach the canal wall surfaces. In contrast, the action of EndoActivator, Eddy, or XP-endo Finisher not only depends on irrigant agitation, but also on agitating action closer to the canal walls without removing dentin.
EndoActivator and Eddy generate intracanal agitation of irrigants though acoustic streaming and cavitation (Bayari, et al., 2017). At all evaluation levels, no significant differences were found between both sonic irrigation/agitation systems, while they were significantly more effective than the control (a conventional needle irrigation technique).
On the other hand, other studies (Takeda, et al., 1999; Saber and Hashem, 2011) have shown that irrigation/agitation systems do not result in significant cleaning differences with the conventional needle irrigation technique. These findings are not supported by the current study or by others (Blank-Gonçalves, et al., 2012).
Sonic agitation systems increase the effectiveness of the irrigating solutions, especially in hard-to-reach areas such as the apical third and, in particular, in the area 1 mm from the WL. The difference between studies may be due to the use of different instruments and/or instrumentation techniques, as well as different experimental models and evaluation protocols.
In the current study, the method used for observation of canal wall surfaces was similar to one used in a previous study (Zmener, et al., 2005). This method allows for examination of both halves of the root, thus examining the entire circumference of the root canal at a predetermined distance from the WL.
The XP-endo Finisher provided significantly cleaner canal walls at all evaluation levels. These results confirmed the claim of the manufacturer stating that the phase transformation of the alloy of the instrument when used at body temperature causes the file to expand, which as a result improves the cleaning activity on wall irregularities.
It should be noted that previous in vitro studies did not use EndoActivator and Eddy at body temperature (Khaord, et al., 2010; Uroz-Torres, et al., 2010; Bayari, et al., 2017; Duque, et al., 2017; Urban, et al., 2017). In this study, the final irrigation/agitation techniques were tested under similar temperature conditions, with the objective to standardize the experimental procedures and to mimic as much as possible the clinical conditions of the oral environment.
Conclusions
Within the limitations of this study, the results indicate that EndoActivator, Eddy, and XP-endo Finisher, along with 5.25% NaOCl and 17% EDTA solution, as a final step in irrigation of root canals have greater potential for DE and SL removal when used in straight root canals.
Although the XP-endo Finisher was superior to EndoActivator, Eddy, and a conventional needle irrigation technique, none of the tested techniques was able to completely clean the root canal walls.
Read what Dr. Gregori M Kurtzman has to add about smear layer removal and laser-enhanced endodontic treatment here.
References
- Abou-Rass M, Piccinino MV. The effectiveness of four clinical irrigation methods on the removal of root canal debris. Oral Surg Oral Med Oral Pathol. 1982;54(3):323-328.
- Ahlquist M, Henningsson O, Hultenby K, Ohlin J. The effectiveness of manual and rotary techniques in the cleaning of root canals: a scanning electron microscopy study. Int Endod J. 2001;34(7):533-537.
- Bao P, Shen Y, Lin J, Haapasalo M. In vitro efficacy of XP-endo Finisher with 2 different protocols on biofilm removal from apical root canals. J Endod. 2017;43(2):321-325.
- Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13(4):147-157.
- Bayari L, Forner L, Villanueva D, Almenar A, Llena C. Elimination of the root canal dentin smear layer by irrigation with new polyamide sonic activated tips. Endodoncia. 2017;35(1):23-33.
- Blank Gonçalves LM, Nabeshima CK, Martins GH, Machado ME. Qualitative analysis of the removal of the smear layer in the apical third of curved roots: conventional irrigation versus activation systems. J Endod. 2011;37(9):1268-1271.
- Caron JI, Nham K, Bronnec F, Machtou P. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod. 2010;36(8):1361-1366.
- Duque JA, Duarte MAH, Canali ICF, Zancan RF, Vivan RR, Bernardes RF, Bramante CM. Comparative effectiveness of new mechanical irrigant agitating devices for debris removal from the canal and isthmus of medial roots of mandibular molars. J Endod. 2017;43:326-331.
- Gu L, Kim JR, Ling J, et al. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35(6):791-804.
- Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J. 2014;216(6):299-303.
- Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod. 1997;23(5):301-306.
- Jiang LM, Lak B, Eijsvogels LM, Wesselink P, van der Sluis LW. Comparison of the cleaning efficacy of different final irrigation techniques. J Endod. 2012;38(6):838-841.
- Khaord P, Amin A, Shah MB, et al. Effectiveness of different irrigation techniques on smear layer removal in apical thirds of mesial root canals of permanent mandibular first molar. A scanning electron microscopic study. J Conserv Dent. 2015;18(4)321-325.
- Kumar VR, Bahuguna N, Manan R. Comparison of efficacy of various root canal irrigation systems in removal of smear layer generated at the apical third. An SEM study. J Conserv Dent. 2015;18(3):252-256.
- Mayer BE, Peters OA, Barbakow F. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores: a scanning electron study. Int Endod J. 2002;35(7):582-589.
- Mendonça DH, Colucci V, Rached Junior FJ, et al. Effects of various irrigation/aspiration protocols on cleaning of flattened root canals. Braz Oral Res. 2015;29:1-9.
- Paqué F, Boessler C, Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int Endod J. 2011;44(2):148-153.
- Peters OA, Barbakow F. Effects of irrigation on debris and smear layer on canal walls prepared by two rotary techniques: a scanning electron microscopic study. J Endod. 2000;26(1):6-10.
- Schafer E, Zapke K. A comparative scanning electron microscopic investigation of the efficacy of manual and automated instrumentation of root canals. J Endod. 2000;26:660-664.
- Svec TA, Harrison JW. Chemomechanical removal of pulpal and dentinal debris with sodium hypochlorite and hydrogen peroxide vs. normal saline solution. J Endod. 1977;3(2):49-53.
- Urban K, Donnermeyer D, Schäfer E, Bürklein S. Canal cleanliness using different irrigation activation systems: a SEM evaluation. Clin Oral Invest. 2017;21(9):2681-2687.
- Uroz-Torres D, Gonzales-Rodriguez MP, Ferraz-Luque CM. Effectiveness of the EndoActivator System in removing the smear layer after root canal instrumentation. J Endod. 2010;36(2):305-311.
- Zehnder M. Root canal irrigants. J Endod. 2006;32(5): 389-398.
- Zmener O, Pameijer CH, Banegas G. Effectiveness in cleaning oval-shaped root canals using Anatomic Endodontic Technology, ProFile and manual instrumentation: a scanning electron microscopic study. Int Endod J. 2005;38(6):356-363.

